Kisspeptin signaling in astrocytes modulates the reproductive axis
- PMID: 38861336
- PMCID: PMC11291270
- DOI: 10.1172/JCI172908
Kisspeptin signaling in astrocytes modulates the reproductive axis
Abstract
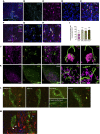
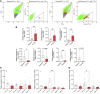
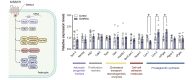
Reproduction is safeguarded by multiple, often cooperative, regulatory networks. Kisspeptin signaling, via KISS1R, plays a fundamental role in reproductive control, primarily by regulation of hypothalamic GnRH neurons. We disclose herein a pathway for direct kisspeptin actions in astrocytes that contributes to central reproductive modulation. Protein-protein interaction and ontology analyses of hypothalamic proteomic profiles after kisspeptin stimulation revealed that glial/astrocyte markers are regulated by kisspeptin in mice. This glial-kisspeptin pathway was validated by the demonstrated expression of Kiss1r in mouse astrocytes in vivo and astrocyte cultures from humans, rats, and mice, where kisspeptin activated canonical intracellular signaling-pathways. Cellular coexpression of Kiss1r with the astrocyte markers GFAP and S100-β occurred in different brain regions, with higher percentage in Kiss1- and GnRH-enriched areas. Conditional ablation of Kiss1r in GFAP-positive cells in the G-KiR-KO mouse altered gene expression of key factors in PGE2 synthesis in astrocytes and perturbed astrocyte-GnRH neuronal appositions, as well as LH responses to kisspeptin and LH pulsatility, as surrogate marker of GnRH secretion. G-KiR-KO mice also displayed changes in reproductive responses to metabolic stress induced by high-fat diet, affecting female pubertal onset, estrous cyclicity, and LH-secretory profiles. Our data unveil a nonneuronal pathway for kisspeptin actions in astrocytes, which cooperates in fine-tuning the reproductive axis and its responses to metabolic stress.
Keywords: Endocrinology; Fertility; Neuroendocrine regulation; Reproductive biochemistry; Reproductive biology.
Figures



Comment in
- Astrocytes: a star emerges in the control of reproductive hormones
Similar articles
-
Astrocytes: a star emerges in the control of reproductive hormones.J Clin Invest. 2024 Aug 1;134(15):e182669. doi: 10.1172/JCI182669. J Clin Invest. 2024. PMID: 39087475 Free PMC article.
-
Connecting nutritional deprivation and pubertal inhibition via GRK2-mediated repression of kisspeptin actions in GnRH neurons.Metabolism. 2022 Apr;129:155141. doi: 10.1016/j.metabol.2022.155141. Epub 2022 Jan 22. Metabolism. 2022. PMID: 35074314 Free PMC article.
-
Deficiency of arcuate nucleus kisspeptin results in postpubertal central hypogonadism.Am J Physiol Endocrinol Metab. 2021 Aug 1;321(2):E264-E280. doi: 10.1152/ajpendo.00088.2021. Epub 2021 Jun 28. Am J Physiol Endocrinol Metab. 2021. PMID: 34181485 Free PMC article.
-
The role of neuropeptides and neurotransmitters on kisspeptin/kiss1r-signaling in female reproduction.J Chem Neuroanat. 2018 Oct;92:71-82. doi: 10.1016/j.jchemneu.2018.07.001. Epub 2018 Jul 6. J Chem Neuroanat. 2018. PMID: 30008384 Review.
-
The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction.Front Endocrinol (Lausanne). 2022 Jun 28;13:925206. doi: 10.3389/fendo.2022.925206. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35837314 Free PMC article. Review.
Cited by
-
Sex and Time of Day Alter the Interactions Between Hypothalamic Glia and the Neural Circuits Controlling Reproduction.Endocrinology. 2025 Mar 24;166(5):bqaf057. doi: 10.1210/endocr/bqaf057. Endocrinology. 2025. PMID: 40111184
-
The role of neonatal kisspeptin in long-term social behavior in mammals.Commun Biol. 2025 Jul 22;8(1):1085. doi: 10.1038/s42003-025-08478-x. Commun Biol. 2025. PMID: 40696196 Free PMC article.
-
Increase of astrocyte apposition on GnRH neurons in early puberty onset induced by high fat diet.J Neuroendocrinol. 2025 Aug;37(8):e70029. doi: 10.1111/jne.70029. Epub 2025 Apr 15. J Neuroendocrinol. 2025. PMID: 40235166 Free PMC article.
-
Astrocytes: a star emerges in the control of reproductive hormones.J Clin Invest. 2024 Aug 1;134(15):e182669. doi: 10.1172/JCI182669. J Clin Invest. 2024. PMID: 39087475 Free PMC article.
-
Intranasal kisspeptin administration rapidly stimulates gonadotropin release in humans.EBioMedicine. 2025 May;115:105689. doi: 10.1016/j.ebiom.2025.105689. Epub 2025 Apr 11. EBioMedicine. 2025. PMID: 40215751 Free PMC article. Clinical Trial.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

