TET1 Regulates Nestin Expression and Human Airway Smooth Muscle Proliferation
- PMID: 38861343
- PMCID: PMC11450309
- DOI: 10.1165/rcmb.2024-0139OC
TET1 Regulates Nestin Expression and Human Airway Smooth Muscle Proliferation
Abstract
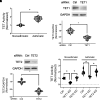
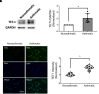
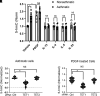
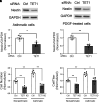
Asthma is characterized by aberrant airway smooth muscle (ASM) proliferation, which increases the thickness of the ASM layer within the airway wall and exacerbates airway obstruction during asthma attacks. The mechanisms that drive ASM proliferation in asthma are not entirely elucidated. Ten-eleven translocation methylcytosine dioxygenase (TET) is an enzyme that participates in the regulation of DNA methylation by catalyzing the hydroxylation of 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC). The generation of 5-hmC disinhibits the gene silencing effect of 5-mC. In this study, TET1 activity and protein were enhanced in asthmatic human ASM cell cultures. Moreover, the concentration of 5-hmC was higher in asthmatic ASM cells than in nonasthmatic ASM cells. Knockdown (KD) of TET1, but not TET2, reduced the concentration of 5-hmC in asthmatic cells. Because the cytoskeletal protein nestin controls cell proliferation by modulating mTOR, we evaluated the effects of TET1 KD on this pathway. TET1 KD reduced nestin expression in ASM cells. In addition, TET1 inhibition alleviated the platelet-derived growth factor-induced phosphorylation of p70S6K, 4E-BP, S6, and Akt. TET1 inhibition also attenuated the proliferation of ASM cells. Taken together, these results suggest that TET1 drives ASM proliferation via the nestin-mTOR axis.
Keywords: DNA methylation; airway smooth muscle; cytoskeletal protein; signal transduction.
Figures


Similar articles
-
Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine.J Biol Chem. 2013 May 10;288(19):13669-74. doi: 10.1074/jbc.C113.464800. Epub 2013 Apr 2. J Biol Chem. 2013. PMID: 23548903 Free PMC article.
-
Ten-eleven translocation 1 (TET1) methylation is associated with childhood asthma and traffic-related air pollution.J Allergy Clin Immunol. 2016 Mar;137(3):797-805.e5. doi: 10.1016/j.jaci.2015.10.021. Epub 2015 Dec 10. J Allergy Clin Immunol. 2016. PMID: 26684294 Free PMC article.
-
MYC deregulates TET1 and TET2 expression to control global DNA (hydroxy)methylation and gene expression to maintain a neoplastic phenotype in T-ALL.Epigenetics Chromatin. 2019 Jul 2;12(1):41. doi: 10.1186/s13072-019-0278-5. Epigenetics Chromatin. 2019. PMID: 31266538 Free PMC article.
-
[Advances in epigenetic regulation of the dioxygenase TET1].Sheng Wu Gong Cheng Xue Bao. 2024 Dec 25;40(12):4351-4364. doi: 10.13345/j.cjb.240191. Sheng Wu Gong Cheng Xue Bao. 2024. PMID: 39722501 Review. Chinese.
-
Epigenetic Function of TET Family, 5-Methylcytosine, and 5-Hydroxymethylcytosine in Hematologic Malignancies.Oncol Res Treat. 2019;42(6):309-318. doi: 10.1159/000498947. Epub 2019 May 3. Oncol Res Treat. 2019. PMID: 31055566 Review.
References
-
- Penn RB. Embracing emerging paradigms of G protein-coupled receptor agonism and signaling to address airway smooth muscle pathobiology in asthma. Naunyn Schmiedebergs Arch Pharmacol . 2008;378:149–169. - PubMed
-
- Ammit AJ, Panettieri RA., Jr Airway smooth muscle cell hyperplasia: a therapeutic target in airway remodeling in asthma? Prog Cell Cycle Res . 2003;5:49–57. - PubMed
-
- Amrani Y, Tliba O, Deshpande DA, Walseth TF, Kannan MS, Panettieri RA., Jr Bronchial hyperresponsiveness: insights into new signaling molecules. Curr Opin Pharmacol . 2004;4:230–234. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

