Sialic Acid Receptor Specificity in Mammary Gland of Dairy Cattle Infected with Highly Pathogenic Avian Influenza A(H5N1) Virus
- PMID: 38861554
- PMCID: PMC11210646
- DOI: 10.3201/eid3007.240689
Sialic Acid Receptor Specificity in Mammary Gland of Dairy Cattle Infected with Highly Pathogenic Avian Influenza A(H5N1) Virus
Abstract
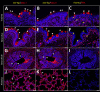
In March 2024, the US Department of Agriculture's Animal and Plant Health Inspection Service reported detection of highly pathogenic avian influenza (HPAI) A(H5N1) virus in dairy cattle in the United States for the first time. One factor that determines susceptibility to HPAI H5N1 infection is the presence of specific virus receptors on host cells; however, little is known about the distribution of the sialic acid (SA) receptors in dairy cattle, particularly in mammary glands. We compared the distribution of SA receptors in the respiratory tract and mammary gland of dairy cattle naturally infected with HPAI H5N1. The respiratory and mammary glands of HPAI H5N1-infected dairy cattle are rich in SA, particularly avian influenza virus-specific SA α2,3-gal. Mammary gland tissues co-stained with sialic acids and influenza A virus nucleoprotein showed predominant co-localization with the virus and SA α2,3-gal. HPAI H5N1 exhibited epitheliotropism within the mammary gland, and we observed rare immunolabeling within macrophages.
Keywords: H5N1; Neu5Ac; United States; dairy cattle; epithelial cell; highly pathogenic avian influenza; influenza; macrophage; mammary gland; respiratory tract; sialic acid; viruses; zoonoses; α2,3-gal; α2,6-gal.
Figures





References
-
- US Department of Agriculture, Animal and Plant Health Inspection Service. Federal and state veterinary, public health agencies share update on HPAI detection in Kansas, Texas dairy herds. 2024. [cited 2024 May 5]. https://www.aphis.usda.gov/news/agency-announcements/federal-state-veter...
-
- US Department of Agriculture, US Department of Health and Human Services. USDA, HHS announce new actions to reduce impact and spread of H5N1. 2024. [cited 2024 May 5]. https://www.usda.gov/media/press-releases/2024/05/10/usda-hhs-announce-n...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

