Ca2+ permeation through C-terminal cleaved, but not full-length human Pannexin1 hemichannels, mediates cell death
- PMID: 38861601
- PMCID: PMC11194574
- DOI: 10.1073/pnas.2405468121
Ca2+ permeation through C-terminal cleaved, but not full-length human Pannexin1 hemichannels, mediates cell death
Abstract
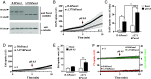
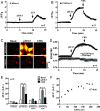
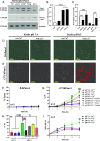
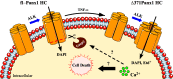
Pannexin1 hemichannels (Panx1 HCs) are found in the membrane of most mammalian cells and communicate the intracellular and extracellular spaces, enabling the passive transfer of ions and small molecules. They are involved in physiological and pathophysiological conditions. During apoptosis, the C-terminal tail of Panx1 is proteolytically cleaved, but the permeability features of hemichannels and their role in cell death remain elusive. To address these topics, HeLa cells transfected with full-length human Panx1 (fl-hPanx1) or C-terminal truncated hPanx1 (Δ371hPanx1) were exposed to alkaline extracellular saline solution, increasing the activity of Panx1 HCs. The Δ371hPanx1 HC was permeable to DAPI and Etd+, but not to propidium iodide, whereas fl-hPanx1 HC was only permeable to DAPI. Furthermore, the cytoplasmic Ca2+ signal increased only in Δ371hPanx1 cells, which was supported by bioinformatics approaches. The influx of Ca2+ through Δ371hPanx1 HCs was necessary to promote cell death up to about 95% of cells, whereas the exposure to alkaline saline solution without Ca2+ failed to induce cell death, and the Ca2+ ionophore A23187 promoted more than 80% cell death even in fl-hPanx1 transfectants. Moreover, cell death was prevented with carbenoxolone or 10Panx1 in Δ371hPanx1 cells, whereas it was undetectable in HeLa Panx1-/- cells. Pretreatment with Ferrostatin-1 and necrostatin-1 did not prevent cell death, suggesting that ferroptosis or necroptosis was not involved. In comparison, zVAD-FMK, a pancaspase inhibitor, reduced death by ~60%, suggesting the involvement of apoptosis. Therefore, alkaline pH increases the activity of Δ371hPanx1HCs, leading to a critical intracellular free-Ca2+ overload that promotes cell death.
Keywords: Ca2+ influx; Pannexin1; cell death; hemichannel.
Conflict of interest statement
Competing interests statement:V.M.B., a reviewer, and J.C.S. were on a retrospective for Michael Bennett together this year (https://pubmed.ncbi.nlm.nih.gov/38278514/).
Figures

Similar articles
-
Poly (I:C)-induced inflammation requires the activation of toll-like receptor 3/Ca2+/CaMKII/pannexin 1-dependent signaling.Theranostics. 2025 Jan 20;15(6):2470-2486. doi: 10.7150/thno.100687. eCollection 2025. Theranostics. 2025. PMID: 39990207 Free PMC article.
-
Pannexin1 channels act downstream of P2X 7 receptors in ATP-induced murine T-cell death.Channels (Austin). 2014;8(2):142-56. doi: 10.4161/chan.28122. Epub 2014 Mar 3. Channels (Austin). 2014. PMID: 24590064 Free PMC article.
-
A physiologic rise in cytoplasmic calcium ion signal increases pannexin1 channel activity via a C-terminus phosphorylation by CaMKII.Proc Natl Acad Sci U S A. 2021 Aug 10;118(32):e2108967118. doi: 10.1073/pnas.2108967118. Proc Natl Acad Sci U S A. 2021. PMID: 34301850 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Calcium Regulation of Connexin Hemichannels.Int J Mol Sci. 2024 Jun 15;25(12):6594. doi: 10.3390/ijms25126594. Int J Mol Sci. 2024. PMID: 38928300 Free PMC article. Review.
Cited by
-
Hemichannels contribute to mitochondrial Ca2+ and morphology alterations evoked by ethanol in astrocytes.Front Cell Dev Biol. 2024 Jul 26;12:1434381. doi: 10.3389/fcell.2024.1434381. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39129788 Free PMC article.
-
Poly (I:C)-induced inflammation requires the activation of toll-like receptor 3/Ca2+/CaMKII/pannexin 1-dependent signaling.Theranostics. 2025 Jan 20;15(6):2470-2486. doi: 10.7150/thno.100687. eCollection 2025. Theranostics. 2025. PMID: 39990207 Free PMC article.
-
Pannexin channels in inflammation and tumorigenesis.Front Cell Dev Biol. 2025 Aug 20;13:1647765. doi: 10.3389/fcell.2025.1647765. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40909173 Free PMC article. Review.
-
NADPH acts as an endogenous P2X7 receptor modulator to gate neuroinflammatory responses of microglia.Acta Pharmacol Sin. 2025 Aug 11. doi: 10.1038/s41401-025-01638-z. Online ahead of print. Acta Pharmacol Sin. 2025. PMID: 40789938
-
Role of Divalent Cations in Infections in Host-Pathogen Interaction.Int J Mol Sci. 2024 Sep 10;25(18):9775. doi: 10.3390/ijms25189775. Int J Mol Sci. 2024. PMID: 39337264 Free PMC article. Review.
References
-
- Penuela S., Gehi R., Laird D. W., The biochemistry and function of pannexin channels. Biochim. Biophys. Acta 1828, 15–22 (2013). - PubMed
MeSH terms
Substances
Grants and funding
- 3210284/ANID | Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT)
- 1231523/ANID | Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT)
- 1201342/ANID | Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT)
- 11241081/ANID | Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

