Progesterone inactivation in decidual stromal cells: A mechanism for inflammation-induced parturition
- PMID: 38861608
- PMCID: PMC11194587
- DOI: 10.1073/pnas.2400601121
Progesterone inactivation in decidual stromal cells: A mechanism for inflammation-induced parturition
Erratum in
-
Correction for DeTomaso et al., Progesterone inactivation in decidual stromal cells: A mechanism for inflammation-induced parturition.Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2415478121. doi: 10.1073/pnas.2415478121. Epub 2024 Aug 26. Proc Natl Acad Sci U S A. 2024. PMID: 39186663 Free PMC article. No abstract available.
Abstract
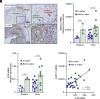
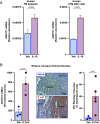
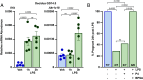
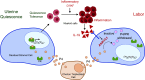
The process of human parturition involves inflammation at the interface where fetal chorion trophoblast cells interact with maternal decidual stromal (DS) cells and maternal immune cells in the decidua (endometrium of pregnancy). This study tested the hypothesis that inflammation at the chorion-decidua interface (CDI) induces labor by negating the capacity for progesterone (P4) to block labor and that this is mediated by inactivation of P4 in DS cells by aldo-keto reductase family 1 member C1 (AKR1C1). In human, Rhesus macaque, and mouse CDI, AKR1C1 expression increased in association with term and preterm labor. In a human DS cell line and in explant cultures of term human fetal membranes containing the CDI, the prolabor inflammatory cytokine, interleukin-1ß (IL-1ß), and media conditioned by LPS-stimulated macrophages increased AKR1C1 expression and coordinately reduced nuclear P4 levels and P4 responsiveness. Loss of P4 responsiveness was overcome by inhibition of AKR1C1 activity, inhibition of AKR1C1 expression, and bypassing AKR1C1 activity with a P4 analog that is not metabolized by AKR1C1. Increased P4 activity in response to AKR1C1 inhibition was prevented by the P4 receptor antagonist RU486. Pharmacologic inhibition of AKR1C1 activity prevented parturition in a mouse model of inflammation-induced preterm parturition. The data suggest that inflammatory stimuli at the CDI drive labor by inducing AKR1C1-mediated P4 inactivation in DS cells and that inhibiting and/or bypassing of AKR1C1-mediated P4 inactivation is a plausible therapeutic strategy to mitigate the risk of inflammation-associated preterm birth.
Keywords: inflammation; parturition; pregnancy; progesterone metabolism.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Young A., et al. , Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol. Reprod. 66, 445–449 (2002). - PubMed
-
- Keelan J. A., et al. , Cytokines, prostaglandins and parturition–A review. Placenta 24, S33–S46 (2003). - PubMed
-
- Osman I., Young A., Jordan F., Greer I. A., Norman J. E., Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J. Soc. Gynecol. Invest. 13, 97–103 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

