Slow proliferation of BAP1-deficient uveal melanoma cells is associated with reduced S6 signaling and resistance to nutrient stress
- PMID: 38861613
- PMCID: PMC11328427
- DOI: 10.1126/scisignal.adn8376
Slow proliferation of BAP1-deficient uveal melanoma cells is associated with reduced S6 signaling and resistance to nutrient stress
Abstract
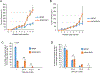
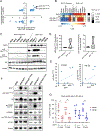
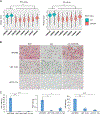
Uveal melanoma (UM) is the deadliest form of eye cancer in adults. Inactivating mutations and/or loss of expression of the gene encoding BRCA1-associated protein 1 (BAP1) in UM tumors are associated with an increased risk of metastasis. To investigate the mechanisms underlying this risk, we explored the functional consequences of BAP1 deficiency. UM cell lines expressing mutant BAP1 grew more slowly than those expressing wild-type BAP1 in culture and in vivo. The ability of BAP1 reconstitution to restore cell proliferation in BAP1-deficient cells required its deubiquitylase activity. Proteomic analysis showed that BAP1-deficient cells had decreased phosphorylation of ribosomal S6 and its upstream regulator, p70S6K1, compared with both wild-type and BAP1 reconstituted cells. In turn, expression of p70S6K1 increased S6 phosphorylation and proliferation of BAP1-deficient UM cells. Consistent with these findings, BAP1 mutant primary UM tumors expressed lower amounts of p70S6K1 target genes, and S6 phosphorylation was decreased in BAP1 mutant patient-derived xenografts (PDXs), which grew more slowly than wild-type PDXs in the liver (the main metastatic site of UM) in mice. BAP1-deficient UM cells were also more resistant to amino acid starvation, which was associated with diminished phosphorylation of S6. These studies demonstrate that BAP1 deficiency slows the proliferation of UM cells through regulation of S6 phosphorylation. These characteristics may be associated with metastasis by ensuring survival during amino acid starvation.
Conflict of interest statement
Figures




Similar articles
-
BAP1 mutant uveal melanoma is stratified by metabolic phenotypes with distinct vulnerability to metabolic inhibitors.Oncogene. 2021 Jan;40(3):618-632. doi: 10.1038/s41388-020-01554-y. Epub 2020 Nov 18. Oncogene. 2021. PMID: 33208912 Free PMC article.
-
Pyruvate dehydrogenase inactivation causes glycolytic phenotype in BAP1 mutant uveal melanoma.Oncogene. 2022 Feb;41(8):1129-1139. doi: 10.1038/s41388-021-02154-0. Epub 2022 Jan 20. Oncogene. 2022. PMID: 35046531 Free PMC article.
-
BAP1 Loss Affords Lipotoxicity Resistance in Uveal Melanoma.Pigment Cell Melanoma Res. 2025 May;38(3):e70021. doi: 10.1111/pcmr.70021. Pigment Cell Melanoma Res. 2025. PMID: 40300851 Free PMC article.
-
[Uveal Melanoma: Molecular and Genetic Mechanisms of Development and Therapeutic Approaches].Mol Biol (Mosk). 2024 Mar-Apr;58(2):189-203. Mol Biol (Mosk). 2024. PMID: 39355878 Review. Russian.
-
A comprehensive review of PRAME and BAP1 in melanoma: Genomic instability and immunotherapy targets.Cell Signal. 2024 Dec;124:111434. doi: 10.1016/j.cellsig.2024.111434. Epub 2024 Sep 24. Cell Signal. 2024. PMID: 39326690 Review.
References
-
- Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123(12):1639–43. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

