Fixed-duration pirtobrutinib plus venetoclax with or without rituximab in relapsed/refractory CLL: the phase 1b BRUIN trial
- PMID: 38861666
- PMCID: PMC11451378
- DOI: 10.1182/blood.2024024510
Fixed-duration pirtobrutinib plus venetoclax with or without rituximab in relapsed/refractory CLL: the phase 1b BRUIN trial
Abstract
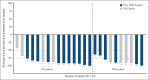
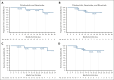
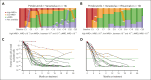
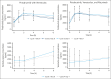
Pirtobrutinib is a highly selective, noncovalent (reversible) Bruton tyrosine kinase inhibitor (BTKi). Patients with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL) were treated with fixed-duration pirtobrutinib plus venetoclax (PV) or pirtobrutinib plus venetoclax and rituximab (PVR) in this phase 1b trial. Prior covalent BTKi therapy was allowed, but not prior treatment with venetoclax. Patients were assigned to receive PV (n = 15) or PVR (n = 10) for 25 cycles. Most patients (68%) had received prior covalent BTKi therapy. At the data cutoff date, the median time on study was 27.0 months for PV and 23.3 months for PVR. Overall response rates were 93.3% (95% confidence interval [CI], 68.1-99.8) for PV and 100% (95% CI, 69.2-100.0) for PVR, with 10 complete responses (PV: 7; PVR: 3). After 12 cycles of treatment, 85.7% (95% CI, 57.2-98.2) of PV and 90.0% (95% CI, 55.5-99.7) of PVR patients achieved undetectable minimal residual disease (<10-4) in peripheral blood. Progression-free survival at 18 months was 92.9% (95% CI, 59.1-99.0) for PV patients and 80.0% (95% CI, 40.9-94.6) for PVR patients. No dose-limiting toxicities were observed during the 5-week assessment period. The most common grade ≥3 adverse events (AEs) for all patients included neutropenia (52%) and anemia (16%). AEs led to dose reduction in 3 patients and discontinuation in 2. In conclusion, fixed-duration PV or PVR was well tolerated and had promising efficacy in patients with R/R CLL, including patients previously treated with a covalent BTKi. This trial was registered at www.clinicaltrials.gov as #NCT03740529.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: L.E.R. has served as a consultant for AbbVie, Ascentage, AstraZeneca, BeiGene, Janssen, Loxo Oncology, Pharmacyclics, Pfizer, and TG Therapeutics and as a continuing medical education speaker for DAVA, Curio, Medscape, and PeerView; holds minority ownership interest in Abbott Laboratories; received travel support from Loxo Oncology; and has received research funding (paid to the institution) from Adaptive Biotechnologies, AstraZeneca, Genentech, AbbVie, Pfizer, Loxo Oncology, Aptose Biosciences, Dren Bio, and Qilu Puget Sound Biotherapeutics. J.A.W. reports personal fees from Loxo Oncology during the conduct of the study; personal fees from Janssen, Pharmacyclics, AstraZeneca, AbbVie, BeiGene, Genentech, Merck, and Newave; and grants from Pharmacyclics, Schrodinger, and AbbVie, outside the submitted work. C.Y.C. reports personal fees from Loxo Oncology during the conduct of the study; grants and personal fees from Roche, AbbVie, and Bristol Myers Squibb; and personal fees from Menarini, Kite, Janssen, Gilead, Genmab, BeiGene, and AstraZeneca outside the submitted work. C.C.C. reports personal fees and honoraria and payment to institution for clinical trial from Loxo Oncology, during the conduct of the study; personal fees from AbbVie, Allogene, AstraZeneca, BeiGene, Genentech, Janssen, MEI Pharma, MingSight, Octapharma, and TG Therapeutics; and payment to institution for clinical trial from AbbVie outside the submitted work. N.N.S. reports personal fees from Loxo Oncology, during the conduct of the study; personal fees and research support and consultancy from Miltenyi Biotec; and personal fees from Incyte, Celgene, Kite, and Verastem, outside the submitted work. M.R.P. reports consultancy, board of director’s membership, or advisory roles for Janssen, EMD Serono, Pfizer, Pharmacyclics, Bayer, Genentech, and Loxo Oncology, during the conduct of the study. N.L. received research funding from Loxo Oncology, Juno, Oncternal, Verastem, TG Therapeutics, MingSight, and Octapharma, and has been in a consulting role for AbbVie, AstraZeneca, BeiGene, Genentech, Celgene, Gilead, Janssen, and Pharmacyclics. D.E.T., B.N., X.Z., N.M., and S.C.M. report full-time employment with Loxo Oncology during the study. C.W. and S.C. report full-time employment with Eli Lilly and Company during the conduct of the study. J.R.B. has served as a consultant for AbbVie, Acerta/AstraZeneca, Alloplex Biotherapeutics, BeiGene, Galapagos NV, Genentech/Roche, Grifols Worldwide Operations, InnoCare Pharma Inc, iOnctura, Kite, Loxo/Lilly, Merck, Numab Therapeutics, Pfizer, and Pharmacyclics, and received research funding from BeiGene, Gilead, iOnctura, Loxo/Lilly, MEI Pharma, and TG Therapeutics. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Pirtobrutinib combinations in CLL.Blood. 2024 Sep 26;144(13):1351-1352. doi: 10.1182/blood.2024025497. Blood. 2024. PMID: 39325480 No abstract available.
References
-
- Hillmen P, Brown JR, Eichhorst BF, et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol. 2020;16(10):517–523. - PubMed
-
- Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–2236. - PubMed
-
- Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

