Reductions of food intake and body weight in diet-induced obese rats following chronic treatment with a monomeric peptide multiagonist
- PMID: 38861891
- PMCID: PMC12285839
- DOI: 10.1016/j.clnu.2024.05.035
Reductions of food intake and body weight in diet-induced obese rats following chronic treatment with a monomeric peptide multiagonist
Abstract
Introduction: While therapies based on endogenous gut peptides such as glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) have been compelling therapeutic agents for obesity and type 2 diabetes (T2D), only a few have achieved long-term weight loss and all have shown significant side-effects, including nausea/malaise and gastrointestinal ailments.
Objective: As the pathophysiology of obesity is driven by dysregulation of multiple, inter-related, pathways, we tested a novel peptide targeting multiple receptors of complementary neurocircuits regulating the controls of energy balance.
Methods: Response to daily injections of GEP44, a GLP-1R and neuropeptide Y1R and Y2R receptor (Y1R/Y2R) triple agonist was tested vs. the GLP-1R agonist liraglutide (LIRA) in diet-induced obese (DIO) male and female rats. Glucose tolerance tests after intraperitoneal injection of glucose (IPGTT) were performed at baseline and after 14-d of treatment in GEP44 treated rats. Other metabolic parameters were assessed in blood at the end of a 28-d intervention.
Results: Upon conclusion at 28-d, body weight reduction compared to vehicle was -15.6%/-11.9% in response to GEP44, vs. -9.7%/-5.1% after LIRA, males, and females, respectively. Significant reductions of cumulative food intake occurred over 28-d in female rats treated with GEP44 (-30%; p < 0.0001), vs. LIRA (-10%), and in male rats GEP44 (-39%; p < 0.0001), vs. LIRA (-20%; p = 0.003). In IPGTTs, a similar stimulation glucose induced insulin secretion was noted in rats treated with GEP44 and LIRA.
Conclusion: The strong reductions of body weight in response to long-term applications of the triple agonist GEP44 confirms the therapeutic potential of targeting multiple receptors for achieving more robust and potentially more sustained improvement of energy balance.
Keywords: Body weight; Calorie intake; Drug intervention; Glucose tolerance; Monomeric multi-receptor agonist; Obesity.
Copyright © 2024 Elsevier Ltd and European Society for Clinical Nutrition and Metabolism. All rights reserved.
Conflict of interest statement
Conflict of interest R.P.D. is a Scientific Advisory Board member of Balchem Corporation, New Hampton, NY, which played no role in the design, execution, or analysis of the results of these studies. R.P.D. is a named author of a patent pursuant to the development of GEP44 that is owned by Syracuse University.
Figures


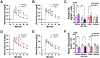
Similar articles
-
The novel chimeric multi-agonist peptide (GEP44) reduces energy intake and body weight in male and female diet-induced obese mice in a glucagon-like peptide-1 receptor-dependent manner.Front Endocrinol (Lausanne). 2024 Jul 22;15:1432928. doi: 10.3389/fendo.2024.1432928. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39104812 Free PMC article.
-
Indirect comparative efficacy and safety of tirzepatide 10 and 15 mg versus semaglutide 2.4 mg for the management of obesity and overweight in patients with type 2 diabetes.Diabetes Obes Metab. 2025 Sep;27(9):4709-4719. doi: 10.1111/dom.16508. Epub 2025 Jun 19. Diabetes Obes Metab. 2025. PMID: 40537987 Free PMC article. Clinical Trial.
-
The quantity, quality and findings of network meta-analyses evaluating the effectiveness of GLP-1 RAs for weight loss: a scoping review.Health Technol Assess. 2025 Jun 25:1-73. doi: 10.3310/SKHT8119. Online ahead of print. Health Technol Assess. 2025. PMID: 40580049 Free PMC article.
-
Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis.Diabetes Obes Metab. 2017 Apr;19(4):524-536. doi: 10.1111/dom.12849. Epub 2017 Feb 17. Diabetes Obes Metab. 2017. PMID: 27981757
-
Selective release of gastrointestinal hormones induced by an orally active GPR39 agonist.Mol Metab. 2021 Jul;49:101207. doi: 10.1016/j.molmet.2021.101207. Epub 2021 Mar 9. Mol Metab. 2021. PMID: 33711555 Free PMC article.
Cited by
-
The Chimeric Peptide (GEP44) Reduces Body Weight and Both Energy Intake and Energy Expenditure in Diet-Induced Obese Rats.bioRxiv [Preprint]. 2025 Feb 22:2025.01.06.631534. doi: 10.1101/2025.01.06.631534. bioRxiv. 2025. Update in: Int J Mol Sci. 2025 Mar 26;26(7):3032. doi: 10.3390/ijms26073032. PMID: 39829931 Free PMC article. Updated. Preprint.
-
The Chimeric Peptide (GEP44) Reduces Body Weight and Both Energy Intake and Energy Expenditure in Diet-Induced Obese Rats.Int J Mol Sci. 2025 Mar 26;26(7):3032. doi: 10.3390/ijms26073032. Int J Mol Sci. 2025. PMID: 40243702 Free PMC article.
References
-
- Afshin A, Reitsma MB, Murray CJL. Health effects of overweight and obesity in 195 Countries. N Engl J Med 2017;377(15):1496–7. - PubMed
-
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2019;157:107843. - PubMed
-
- Bays H, Dujovne C. Anti-obesity drug development. Expet Opin Invest Drugs 2002;11(9):1189–204. - PubMed
-
- Neoh SL, Sumithran P, Haywood CJ, Houlihan CA, Lee FT, Proietto J. Combination phentermine and topiramate for weight maintenance: the first Australian experience. Med J Aust 2014;201(4):224–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

