Blinding Assessments in Neonatal Ventilation Meta-Analyses: A Systematic Meta-Epidemiological Review
- PMID: 38861954
- PMCID: PMC11633896
- DOI: 10.1159/000539203
Blinding Assessments in Neonatal Ventilation Meta-Analyses: A Systematic Meta-Epidemiological Review
Abstract
Introduction: Randomization and blinding are generally important in randomized trials. In neonatology, blinding of ventilation strategies is unfeasible if not impossible and we hypothesized that its importance has been overestimated, while the peculiarities of the neonatal patient and the specific outcomes have not been considered.
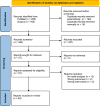
Methods: For this meta-epidemiological review, we searched PubMed and Scopus databases in November 2023. We included all meta-analyses focusing on ventilation, published in past 5 years, and reporting either mortality or bronchopulmonary dysplasia (BPD) as an outcome. We extracted the information about how the authors had analyzed risk of bias and evidence certainty.
Results: We screened 494 abstracts and included 40 meta-analyses. Overall, 13 of the 40 reviews assessed blinding properly. Australian and European authors were most likely to perform correct assessment of the blinding (p = 0.03) and the use of RoB 2.0 tool was also associated with proper assessment (p < 0.001). In multivariate regression, the use of RoB 2.0 was the only factor associated with a proper assessment (Beta 0.57 [95% confidence interval: 0.29-0.99]). GRADE ratings were performed in 25 reviews, and the authors downgraded the evidence certainty due to risk of bias in 19 of these and none of these reviews performed the blinding assessment correctly.
Conclusion: In past neonatal evidence syntheses, the role of blinding has been mostly overestimated, which has led to downgrading of evidence certainty. Objective outcomes (such as mortality and BPD) do not need to be downgraded due to lack of blinding, as the knowledge of the received intervention does not influence the outcome assessment.
Keywords: Blinding assessment; Meta-analysis; Meta-epidemiology; Systematic review; Ventilation.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
I.K. and K.R. have none to report. D.D.L. received consultancy and lecture fees from Chiesi Farmaceutici, Getinge, Vyaire, Radiometer, Medtronic, Astra Zeneca, Boehringer Ingelheim, Airway Therapeutics, Natus, Masimo; he also has equity options from Ophirex Ltd. All these were unrelated to the present work. M.R.G. received a lecture fee from Sanofi, unrelated to this work.
Figures
Comment in
-
Lack of Blinding May Affect Objective Outcomes in Trials on Neonatal Ventilation.Neonatology. 2024;121(6):791-792. doi: 10.1159/000540604. Epub 2024 Aug 16. Neonatology. 2024. PMID: 39154644 No abstract available.
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
A meta-epidemiological study to examine the association between bias and treatment effects in neonatal trials.Evid Based Child Health. 2014 Dec;9(4):1052-9. doi: 10.1002/ebch.1985. Evid Based Child Health. 2014. PMID: 25504975
-
Compelling evidence from meta-epidemiological studies demonstrates overestimation of effects in randomized trials that fail to optimize randomization and blind patients and outcome assessors.J Clin Epidemiol. 2024 Jan;165:111211. doi: 10.1016/j.jclinepi.2023.11.001. Epub 2023 Nov 7. J Clin Epidemiol. 2024. PMID: 37939743
-
Are Neonatal Trials Better Conducted and Reported over the Last 6 Decades? An Analysis on Their Risk-of-Bias Status in Cochrane Reviews.Neonatology. 2019;116(2):123-131. doi: 10.1159/000497423. Epub 2019 May 20. Neonatology. 2019. PMID: 31108494
-
Interventions to Prevent Bronchopulmonary Dysplasia in Preterm Neonates: An Umbrella Review of Systematic Reviews and Meta-analyses.JAMA Pediatr. 2022 May 1;176(5):502-516. doi: 10.1001/jamapediatrics.2021.6619. JAMA Pediatr. 2022. PMID: 35226067
Cited by
-
Evaluating the Performance of ChatGPT-4o in Risk of Bias Assessments.J Evid Based Med. 2024 Dec;17(4):700-702. doi: 10.1111/jebm.12662. Epub 2024 Dec 15. J Evid Based Med. 2024. PMID: 39676337 Free PMC article. No abstract available.
-
Evidence certainty in neonatology-a meta-epidemiological analysis of Cochrane reviews.Eur J Pediatr. 2025 Feb 11;184(2):191. doi: 10.1007/s00431-025-06023-w. Eur J Pediatr. 2025. PMID: 39934466 Free PMC article.
-
ChatGPT-4o in Risk-of-Bias Assessments in Neonatology: A Validity Analysis.Neonatology. 2025;122(3):360-365. doi: 10.1159/000544857. Epub 2025 Feb 25. Neonatology. 2025. PMID: 39999815 Free PMC article.
References
-
- Wallace SS, Barak G, Truong G, Parker MW. Hierarchy of evidence within the medical literature. Hosp Pediatr. 2022;12(8):745–50. - PubMed
-
- Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet Lond Engl. 2002;359(9307):696–700. - PubMed
-
- Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. . A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35(1):49–60. - PubMed
-
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous