Electrical rejuvenation of chronically implanted macroelectrodes in nonhuman primates
- PMID: 38862007
- PMCID: PMC11302379
- DOI: 10.1088/1741-2552/ad5703
Electrical rejuvenation of chronically implanted macroelectrodes in nonhuman primates
Abstract
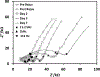
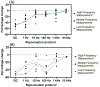
Objective.Electrodes chronically implanted in the brain undergo complex changes over time that can lower the signal to noise ratio (SNR) of recorded signals and reduce the amount of energy delivered to the tissue during therapeutic stimulation, both of which are relevant for the development of robust, closed-loop control systems. Several factors have been identified that link changes in the electrode-tissue interface (ETI) to increased impedance and degraded performance in micro- and macro-electrodes. Previous studies have demonstrated that brief pulses applied every few days can restore SNR to near baseline levels during microelectrode recordings in rodents, a process referred to as electrical rejuvenation. However, electrical rejuvenation has not been tested in clinically relevant macroelectrode designs in large animal models, which could serve as preliminary data for translation of this technique. Here, several variations of this approach were tested to characterize parameters for optimization.Approach. Alternating-current (AC) and direct-current (DC) electrical rejuvenation methods were explored in three electrode types, chronically implanted in two adult male nonhuman primates (NHP) (Macaca mulatta), which included epidural electrocorticography (ECoG) electrodes and penetrating deep-brain stimulation (DBS) electrodes. Electrochemical impedance spectroscopy (EIS) was performed before and after each rejuvenation paradigm as a gold standard measure of impedance, as well as at subsequent intervals to longitudinally track the evolution of the ETI. Stochastic error modeling was performed to assess the standard deviation of the impedance data, and consistency with the Kramers-Kronig relations was assessed to evaluate the stationarity of EIS measurement.Main results. AC and DC rejuvenation were found to quickly reduce impedance and minimize the tissue component of the ETI on all three electrode types, with DC and low-frequency AC producing the largest impedance drops and reduction of the tissue component in Nyquist plots. The effects of a single rejuvenation session were found to last from several days to over 1 week, and all rejuvenation pulses induced no observable changes to the animals' behavior.Significance. These results demonstrate the effectiveness of electrical rejuvenation for diminishing the impact of chronic ETI changes in NHP with clinically relevant macroelectrode designs.
Keywords: chronic implant; electrochemical impedance spectroscopy; electrode; foreign-body response; nonhuman primate; rejuvenation.
© 2024 IOP Publishing Ltd.
Figures
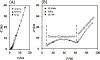
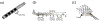

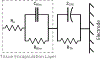





Similar articles
-
Quantifying physical degradation alongside recording and stimulation performance of 980 intracortical microelectrodes chronically implanted in three humans for 956-2130 days.Acta Biomater. 2025 May 15;198:188-206. doi: 10.1016/j.actbio.2025.02.030. Epub 2025 Mar 2. Acta Biomater. 2025. PMID: 40037510
-
Neuromuscular electrical stimulation (NMES) for patellofemoral pain syndrome.Cochrane Database Syst Rev. 2017 Dec 12;12(12):CD011289. doi: 10.1002/14651858.CD011289.pub2. Cochrane Database Syst Rev. 2017. PMID: 29231243 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Electric fans for reducing adverse health impacts in heatwaves.Cochrane Database Syst Rev. 2012 Jul 11;2012(7):CD009888. doi: 10.1002/14651858.CD009888.pub2. Cochrane Database Syst Rev. 2012. PMID: 22786530 Free PMC article.
References
-
- Agarwal P, Crisalle OD, Orazem ME, & Garcia-Rubio LH (1995). Application of Measurement Models to Impedance Spectroscopy: II. Determination of the Stochastic Contribution to the Error Structure (Journal of The Electrochemical Society, pp. 4149–4158).
-
- Agarwal P, Orazem ME, & Garcia-Rubio LH (1992). Measurement Models for Electrochemical Impedance Spectroscopy: I. Demonstration of Applicability. Journal of The Electrochemical Society, 139(7), 1917–1927. 10.1149/1.2069522 - DOI
-
- Ansó J, Benjaber M, Parks B, Parker S, Oehrn CR, Petrucci M, Gilron R, Little S, Wilt R, Bronte-Stewart H, Gunduz A, Borton D, Starr PA, & Denison T (2022). Concurrent stimulation and sensing in bi-directional brain interfaces: a multi-site translational experience. Journal of Neural Engineering, 19(2), 10.1088/1741-2552/ac59a3. 10.1088/1741-2552/ac59a3 - DOI - DOI - PMC - PubMed
-
- Baker JL, Ryou J-W, Wei XF, Butson CR, Schiff ND, & Purpura KP (2016). Robust modulation of arousal regulation, performance, and frontostriatal activity through central thalamic deep brain stimulation in healthy nonhuman primates. Journal of Neurophysiology, 116(5), 2383–2404. 10.1152/jn.01129.2015 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
