3-Position Biaryl Endochin-like Quinolones with Enhanced Antimalarial Performance
- PMID: 38862127
- PMCID: PMC11245370
- DOI: 10.1021/acsinfecdis.4c00140
3-Position Biaryl Endochin-like Quinolones with Enhanced Antimalarial Performance
Abstract
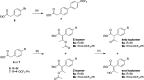
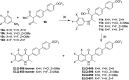
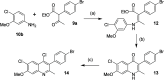
ELQ-300 is a potent antimalarial drug with activity against blood, liver, and vector stages of the disease. A prodrug, ELQ-331, exhibits reduced crystallinity and improved in vivo efficacy in preclinical testing, and currently, it is in the developmental pipeline for once-a-week dosing for oral prophylaxis against malaria. Because of the high cost of developing a new drug for human use and the high risk of drug failure, it is prudent to have a back-up plan in place. Here we describe ELQ-596, a member of a new subseries of 3-biaryl-ELQs, with enhanced potency in vitro against multidrug-resistant Plasmodium falciparum parasites. ELQ-598, a prodrug of ELQ-596 with diminished crystallinity, is more effective vs murine malaria than its progenitor ELQ-331 by 4- to 10-fold, suggesting that correspondingly lower doses could be used to protect and cure humans of malaria. With a longer bloodstream half-life in mice compared to its progenitor, ELQ-596 highlights a novel series of next-generation ELQs with the potential for once-monthly dosing for protection against malaria infection. Advances in the preparation of 3-biaryl-ELQs are presented along with preliminary results from experiments to explore key structure-activity relationships for drug potency, selectivity, pharmacokinetics, and safety.
Keywords: antimalarial drug; next-generation ELQs; oral prophylaxis; structure−activity relationships.
Conflict of interest statement
The authors declare the following competing financial interest(s): OHSU and S.P., R.W.W., R.A.D., K.L, Y.L., A.N., J.X.K., M.J.S., P.H.A., J.S.D., and M.K.R. have a financial interest in a company that may have a commercial interest in the results of this research and technology. A patent application on the intellectual property described herein has been filed by OHSU, WO2023239811 A1.
Figures




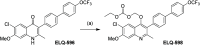

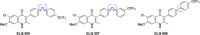
Similar articles
-
Alkoxycarbonate Ester Prodrugs of Preclinical Drug Candidate ELQ-300 for Prophylaxis and Treatment of Malaria.ACS Infect Dis. 2017 Oct 13;3(10):728-735. doi: 10.1021/acsinfecdis.7b00062. Epub 2017 Sep 27. ACS Infect Dis. 2017. PMID: 28927276 Free PMC article.
-
Optimization of endochin-like quinolones for antimalarial activity.Exp Parasitol. 2011 Feb;127(2):545-51. doi: 10.1016/j.exppara.2010.10.016. Epub 2010 Oct 30. Exp Parasitol. 2011. PMID: 21040724 Free PMC article.
-
Role of Trifluoromethyl Substitution in Design of Antimalarial Quinolones: a Comprehensive Review.Top Curr Chem (Cham). 2019 Mar 5;377(2):9. doi: 10.1007/s41061-019-0234-7. Top Curr Chem (Cham). 2019. PMID: 30835005 Review.
-
Synthesis of Deuterated Endochin-Like Quinolones.J Labelled Comp Radiopharm. 2024 May 15;67(5):186-196. doi: 10.1002/jlcr.4092. Epub 2024 Apr 25. J Labelled Comp Radiopharm. 2024. PMID: 38661253 Free PMC article.
-
Hybrid molecules with potential in vitro antiplasmodial and in vivo antimalarial activity against drug-resistant Plasmodium falciparum.Med Res Rev. 2020 May;40(3):931-971. doi: 10.1002/med.21643. Epub 2019 Nov 6. Med Res Rev. 2020. PMID: 31692025 Review.
Cited by
-
Effectiveness of Two New Endochin-like Quinolones, ELQ-596 and ELQ-650, in Experimental Mouse Models of Human Babesiosis.ACS Infect Dis. 2024 Apr 12;10(4):1405-1413. doi: 10.1021/acsinfecdis.4c00143. Epub 2024 Apr 2. ACS Infect Dis. 2024. PMID: 38563132 Free PMC article.
References
-
- WHO World malaria report 2021; World Health Organization: Geneva, 2021.
-
- Dondorp A. M.; Nosten F.; Yi P.; Das D.; Phyo A. P.; Tarning J.; Lwin K. M.; Ariey F.; Hanpithakpong W.; Lee S. J.; Ringwald P.; Silamut K.; Imwong M.; Chotivanich K.; Lim P.; Herdman T.; An S. S.; Yeung S.; Singhasivanon P.; Day N. P.; Lindegardh N.; Socheat D.; White N. J. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J. Med. 2009, 361 (5), 455–467. 10.1056/NEJMoa0808859. - DOI - PMC - PubMed
-
- Balikagala B.; Fukuda N.; Ikeda M.; Katuro O. T.; Tachibana S. I.; Yamauchi M.; Opio W.; Emoto S.; Anywar D. A.; Kimura E.; Palacpac N. M. Q.; Odongo-Aginya E. I.; Ogwang M.; Horii T.; Mita T. Evidence of Artemisinin-Resistant Malaria in Africa. N Engl J. Med. 2021, 385 (13), 1163–1171. 10.1056/NEJMoa2101746. - DOI - PubMed
-
- Macintyre F.; Ramachandruni H.; Burrows J. N.; Holm R.; Thomas A.; Mohrle J. J.; Duparc S.; Hooft van Huijsduijnen R.; Greenwood B.; Gutteridge W. E.; Wells T. N. C.; Kaszubska W. Injectable anti-malarials revisited: discovery and development of new agents to protect against malaria. Malar J. 2018, 17 (1), 402. 10.1186/s12936-018-2549-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

