Glial metabolism versatility regulates mushroom body-driven behavioral output in Drosophila
- PMID: 38862167
- PMCID: PMC11199944
- DOI: 10.1101/lm.053823.123
Glial metabolism versatility regulates mushroom body-driven behavioral output in Drosophila
Abstract
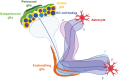
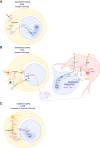
Providing metabolic support to neurons is now recognized as a major function of glial cells that is conserved from invertebrates to vertebrates. However, research in this field has focused for more than two decades on the relevance of lactate and glial glycolysis for neuronal energy metabolism, while overlooking many other facets of glial metabolism and their impact on neuronal physiology, circuit activity, and behavior. Here, we review recent work that has unveiled new features of glial metabolism, especially in Drosophila, in the modulation of behavioral traits involving the mushroom bodies (MBs). These recent findings reveal that spatially and biochemically distinct modes of glucose-derived neuronal fueling are implemented within the MB in a memory type-specific manner. In addition, cortex glia are endowed with several antioxidant functions, whereas astrocytes can serve as pro-oxidant agents that are beneficial to redox signaling underlying long-term memory. Finally, glial fatty acid oxidation seems to play a dual fail-safe role: first, as a mode of energy production upon glucose shortage, and, second, as a factor underlying the clearance of excessive oxidative load during sleep. Altogether, these integrated studies performed in Drosophila indicate that glial metabolism has a deterministic role on behavior.
© 2024 Basu et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
Similar articles
-
Glial glucose fuels the neuronal pentose phosphate pathway for long-term memory.Cell Rep. 2021 Aug 24;36(8):109620. doi: 10.1016/j.celrep.2021.109620. Cell Rep. 2021. PMID: 34433052 Free PMC article.
-
Analysis of Glial Distribution in Drosophila Adult Brains.Neurosci Bull. 2016 Apr;32(2):162-70. doi: 10.1007/s12264-016-0014-0. Epub 2016 Jan 25. Neurosci Bull. 2016. PMID: 26810782 Free PMC article.
-
Glycolytically impaired Drosophila glial cells fuel neural metabolism via β-oxidation.Nat Commun. 2023 May 24;14(1):2996. doi: 10.1038/s41467-023-38813-x. Nat Commun. 2023. PMID: 37225684 Free PMC article.
-
Drosophila Central Nervous System Glia.Cold Spring Harb Perspect Biol. 2015 Feb 26;7(11):a020552. doi: 10.1101/cshperspect.a020552. Cold Spring Harb Perspect Biol. 2015. PMID: 25722465 Free PMC article. Review.
-
Skewing information flow through pre- and postsynaptic plasticity in the mushroom bodies of Drosophila.Learn Mem. 2024 Jun 14;31(5):a053919. doi: 10.1101/lm.053919.124. Print 2024 May. Learn Mem. 2024. PMID: 38876487 Free PMC article. Review.
Cited by
-
What do the mushroom bodies do for the insect brain? Twenty-five years of progress.Learn Mem. 2024 Jun 11;31(5):a053827. doi: 10.1101/lm.053827.123. Print 2024 May. Learn Mem. 2024. PMID: 38862175 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
