Roles of feedback and feed-forward networks of dopamine subsystems: insights from Drosophila studies
- PMID: 38862171
- PMCID: PMC11199952
- DOI: 10.1101/lm.053807.123
Roles of feedback and feed-forward networks of dopamine subsystems: insights from Drosophila studies
Abstract
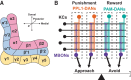
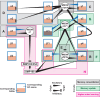
Across animal species, dopamine-operated memory systems comprise anatomically segregated, functionally diverse subsystems. Although individual subsystems could operate independently to support distinct types of memory, the logical interplay between subsystems is expected to enable more complex memory processing by allowing existing memory to influence future learning. Recent comprehensive ultrastructural analysis of the Drosophila mushroom body revealed intricate networks interconnecting the dopamine subsystems-the mushroom body compartments. Here, we review the functions of some of these connections that are beginning to be understood. Memory consolidation is mediated by two different forms of network: A recurrent feedback loop within a compartment maintains sustained dopamine activity required for consolidation, whereas feed-forward connections across compartments allow short-term memory formation in one compartment to open the gate for long-term memory formation in another compartment. Extinction and reversal of aversive memory rely on a similar feed-forward circuit motif that signals omission of punishment as a reward, which triggers plasticity that counteracts the original aversive memory trace. Finally, indirect feed-forward connections from a long-term memory compartment to short-term memory compartments mediate higher-order conditioning. Collectively, these emerging studies indicate that feedback control and hierarchical connectivity allow the dopamine subsystems to work cooperatively to support diverse and complex forms of learning.
© 2024 Davidson and Hige; Published by Cold Spring Harbor Laboratory Press.
Figures
Similar articles
-
Dopamine-mediated interactions between short- and long-term memory dynamics.Nature. 2024 Oct;634(8036):1141-1149. doi: 10.1038/s41586-024-07819-w. Epub 2024 Jul 22. Nature. 2024. PMID: 39038490 Free PMC article.
-
Hierarchical architecture of dopaminergic circuits enables second-order conditioning in Drosophila.Elife. 2023 Jan 24;12:e79042. doi: 10.7554/eLife.79042. Elife. 2023. PMID: 36692262 Free PMC article.
-
A GABAergic Feedback Shapes Dopaminergic Input on the Drosophila Mushroom Body to Promote Appetitive Long-Term Memory.Curr Biol. 2018 Jun 4;28(11):1783-1793.e4. doi: 10.1016/j.cub.2018.04.040. Epub 2018 May 17. Curr Biol. 2018. PMID: 29779874 Free PMC article.
-
Dopamine reveals neural circuit mechanisms of fly memory.Trends Neurosci. 2010 Oct;33(10):457-64. doi: 10.1016/j.tins.2010.07.001. Epub 2010 Aug 10. Trends Neurosci. 2010. PMID: 20701984 Free PMC article. Review.
-
Drosophila memory: dopamine signals punishment?Curr Biol. 2005 Nov 22;15(22):R932-4. doi: 10.1016/j.cub.2005.10.058. Curr Biol. 2005. PMID: 16303554 Review.
Cited by
-
What do the mushroom bodies do for the insect brain? Twenty-five years of progress.Learn Mem. 2024 Jun 11;31(5):a053827. doi: 10.1101/lm.053827.123. Print 2024 May. Learn Mem. 2024. PMID: 38862175 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
