Sensory encoding and memory in the mushroom body: signals, noise, and variability
- PMID: 38862174
- PMCID: PMC11199953
- DOI: 10.1101/lm.053825.123
Sensory encoding and memory in the mushroom body: signals, noise, and variability
Abstract
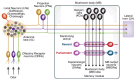
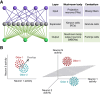
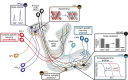
To survive in changing environments, animals need to learn to associate specific sensory stimuli with positive or negative valence. How do they form stimulus-specific memories to distinguish between positively/negatively associated stimuli and other irrelevant stimuli? Solving this task is one of the functions of the mushroom body, the associative memory center in insect brains. Here we summarize recent work on sensory encoding and memory in the Drosophila mushroom body, highlighting general principles such as pattern separation, sparse coding, noise and variability, coincidence detection, and spatially localized neuromodulation, and placing the mushroom body in comparative perspective with mammalian memory systems.
© 2024 Parnas et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Albus JS. 1971. A theory of cerebellar function. Math Biosci 10: 25–61. 10.1016/0025-5564(71)90051-4 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
