Target Trial Emulation: Improving the Quality of Observational Studies in Inflammatory Bowel Disease Using the Principles of Randomized Trials
- PMID: 38862178
- PMCID: PMC11879188
- DOI: 10.1093/ibd/izae131
Target Trial Emulation: Improving the Quality of Observational Studies in Inflammatory Bowel Disease Using the Principles of Randomized Trials
Abstract
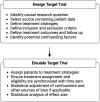
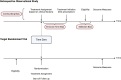
The past decade has seen a substantial increase in the number of randomized controlled trials (RCTs) conducted in inflammatory bowel disease (IBD). Randomized controlled trials are the gold standard method for generating robust evidence of drug safety and efficacy but are expensive, time-consuming, and may have ethical implications. Observational studies in IBD are often used to fill the gaps in evidence but are typically hindered by significant bias. There are several approaches for making statistical inferences from observational data with some that focus on study design and others on statistical techniques. Target trial emulation is an emerging methodological process that aims to bridge this gap and improve the quality of observational studies by applying the principles of an ideal, or "target," randomized trial to routinely collected clinical data. There has been a rapid expansion of observational studies that have emulated trials over the past 5 years in other medical fields, but this has yet to be adopted in gastroenterology and IBD. The wealth of nonrandomized clinical data available through electronic health records, patient registries, and administrative health databases afford innumerable hypothesis-generating opportunities for IBD research. This review outlines the principles of target trial emulation, discusses the merits to IBD observational studies in reducing the most common biases and improving confidence in causality, and details the caveats of using this approach.
Keywords: causal inference; inflammatory bowel disease; randomized controlled trial; target trial.
Plain language summary
Target trial emulation uses observational data to mimic the principles of an ideal or “target” randomized trial. This framework offers several opportunities to strengthen the quality of observational research in inflammatory bowel disease by reducing common sources of bias.
© 2024 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
S.H. has served as a speaker, a consultant, an advisory board member, and/or has received travel grants from Pfizer, Janssen, AbbVie, Takeda, Ferring, Lilly, Pharmacosmos, Dr Falk Pharma, and Galapagos.
S.D. has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alfa Wasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson & Johnson, Millennium/Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB, and Vifor.
L.P.B. reports consulting fees from Abbvie, Abivax, Adacyte, Alimentiv, Amgen, Applied Molecular Transport, Arena, Banook, Biogen, BMS, Celltrion, Connect Biopharm, Cytoki Pharma, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead, Gossamer Bio, GSK, IAC Image Analysis, Index Pharmaceuticals, Inotrem, Janssen, Lilly, Medac, Mopac, Morphic, MSD, Nordic Pharma, Novartis, Oncodesign Precision Medicine, ONO Pharma, OSE Immunotherapeuthics, Pandion Therapeuthics, Par’ Immune, Pfizer, Prometheus, Protagonist, Roche, Samsung, Sandoz, Sanofi, Satisfay, Takeda, Telavant, Theravance, Thermo Fischer, Tigenix, Tillots, Viatris, Vectivbio, Ventyx, Ysopia. LPB reports grants from Celltrion, Fresenius Kabi, Medac, MSD, Takeda. L.P.B. reports lecture fees from Abbvie, Alfa Sigma, Amgen, Arena, Biogen, Celltrion, Ferring, Galapagos, Genentech, Gilead, Janssen, Kern Pharma, Lilly, Medac, MSD, Nordic Pharma, Pfizer, Sandoz, Takeda, Tillots, Viatris. L.P.B. reports travel support fees from Abbvie, Alfa Sigma, Amgen, Celltrion, Connect Biopharm, Ferring, Galapagos, Genentech, Gilead, Gossamer Bio, Janssen, Lilly, Medac, Morphic, MSD, Pfizer, Sandoz, Takeda, Thermo Fischer, and Tillots.
Figures
References
-
- Hariton E, Locascio JJ.. Randomised controlled trials—the gold standard for effectiveness research. BJOG. 2018;125(13):1716. doi: https://doi.org/ 10.1111/1471-0528.15199 - DOI - PMC - PubMed
-
- Truelove SC, Witts LJ.. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2(4947):1041-1048. doi: https://doi.org/ 10.1136/bmj.2.4947.1041 - DOI - PMC - PubMed
-
- Bykov K, Patorno E, D'Andrea E, et al. Prevalence of avoidable and bias-inflicting methodological pitfalls in real-world studies of medication safety and effectiveness. Clin Pharmacol Ther. 2022;111(1):209-217. doi: https://doi.org/ 10.1002/cpt.2364 - DOI - PMC - PubMed
-
- Piovani D, Pansieri C, Peyrin-Biroulet L, Danese S, Bonovas S.. Confounding and bias in observational studies in inflammatory bowel disease: a meta-epidemiological study. Aliment Pharmacol Ther. 2021;53(6):712-721. doi: https://doi.org/ 10.1111/apt.16222 - DOI - PubMed
-
- D’Agostino RB, D'Agostino RB.. Estimating treatment effects using observational data. JAMA. 2007;297(3):314-316. doi: https://doi.org/ 10.1001/jama.297.3.314 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

