Effects of prime-boost strategies on the protective efficacy and immunogenicity of a PLGA (85:15)-encapsulated Chlamydia recombinant MOMP nanovaccine
- PMID: 38862192
- PMCID: PMC11186516
- DOI: 10.1093/femspd/ftae004
Effects of prime-boost strategies on the protective efficacy and immunogenicity of a PLGA (85:15)-encapsulated Chlamydia recombinant MOMP nanovaccine
Abstract
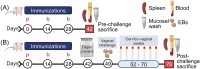
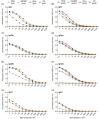
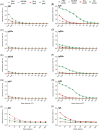
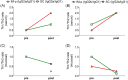
To begin to optimize the immunization routes for our reported PLGA-rMOMP nanovaccine [PLGA-encapsulated Chlamydia muridarum (Cm) recombinant major outer membrane protein (rMOMP)], we compared two prime-boost immunization strategies [subcutaneous (SC) and intramuscular (IM-p) prime routes followed by two SC-boosts)] to evaluate the nanovaccine-induced protective efficacy and immunogenicity in female BALB/c mice. Our results showed that mice immunized via the SC and IM-p routes were protected against a Cm genital challenge by a reduction in bacterial burden and with fewer bacteria in the SC mice. Protection of mice correlated with rMOMP-specific Th1 (IL-2 and IFN-γ) and not Th2 (IL-4, IL-9, and IL-13) cytokines, and CD4+ memory (CD44highCD62Lhigh) T-cells, especially in the SC mice. We also observed higher levels of IL-1α, IL-6, IL-17, CCL-2, and G-CSF in SC-immunized mice. Notably, an increase of cytokines/chemokines was seen after the challenge in the SC, IM-p, and control mice (rMOMP and PBS), suggesting a Cm stimulation. In parallel, rMOMP-specific Th1 (IgG2a and IgG2b) and Th2 (IgG1) serum, mucosal, serum avidity, and neutralizing antibodies were more elevated in SC than in IM-p mice. Overall, the homologous SC prime-boost immunization of mice induced enhanced cellular and antibody responses with better protection against a genital challenge compared to the heterologous IM-p.
Keywords: Chlamydia; l-lactic-co-glycolide)] nanoparticles; IFN-γ; PLGA [poly (d; immunization routes.
© The Author(s) 2024. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

