Neoadjuvant toripalimab combined with axitinib in patients with locally advanced clear cell renal cell carcinoma: a single-arm, phase II trial
- PMID: 38862251
- PMCID: PMC11168135
- DOI: 10.1136/jitc-2023-008475
Neoadjuvant toripalimab combined with axitinib in patients with locally advanced clear cell renal cell carcinoma: a single-arm, phase II trial
Abstract
Background: A combination of axitinib and immune checkpoint inhibitors (ICIs) demonstrated promising efficacy in the treatment of advanced renal cell carcinoma (RCC). This study aims to prospectively evaluate the safety, efficacy, and biomarkers of neoadjuvant toripalimab plus axitinib in non-metastatic clear cell RCC.
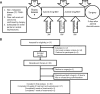
Methods: This is a single-institution, single-arm phase II clinical trial. Patients with non-metastatic biopsy-proven clear cell RCC (T2-T3N0-1M0) are enrolled. Patients will receive axitinib 5 mg twice daily combined with toripalimab 240 mg every 3 weeks (three cycles) for up to 12 weeks. Patients then will receive partial (PN) or radical nephrectomy (RN) after neoadjuvant therapy. The primary endpoint is objective response rate (ORR). Secondary endpoints include disease-free survival, safety, and perioperative complication rate. Predictive biomarkers are involved in exploratory analysis.
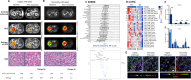
Results: A total of 20 patients were enrolled in the study, with 19 of them undergoing surgery. One patient declined surgery. The primary endpoint ORR was 45%. The posterior distribution of πORR had a mean of 0.44 (95% credible intervals: 0.24-0.64), meeting the predefined primary endpoint with an ORR of 32%. Tumor shrinkage was observed in 95% of patients prior to nephrectomy. Furthermore, four patients achieved a pathological complete response. Grade ≥3 adverse events occurred in 25% of patients, including hypertension, hyperglycemia, glutamic pyruvic transaminase/glutamic oxaloacetic transaminase (ALT/AST) increase, and proteinuria. Postoperatively, one grade 4a and eight grade 1-2 complications were noted. In comparison to patients with stable disease, responders exhibited significant differences in immune factors such as Arginase 1(ARG1), Melanoma antigen (MAGEs), Dendritic Cell (DC), TNF Superfamily Member 13 (TNFSF13), Apelin Receptor (APLNR), and C-C Motif Chemokine Ligand 3 Like 1 (CCL3-L1). The limitation of this trial was the small sample size.
Conclusion: Neoadjuvant toripalimab combined with axitinib shows encouraging activity and acceptable toxicity in locally advanced clear cell RCC and warrants further study.
Trial registration number: clinicaltrials.gov, NCT04118855.
Keywords: Immune Checkpoint Inhibitors; Renal Cell Carcinoma.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SC and YL, employees of Shanghai Junshi Biosciences Inc. WZ, JZ, and DZ, employees of 3D Medicines Inc. Other authors declare no potential conflicts of interest.
Figures


Similar articles
-
Toripalimab plus axitinib versus sunitinib as first-line treatment for advanced renal cell carcinoma: RENOTORCH, a randomized, open-label, phase III study.Ann Oncol. 2024 Feb;35(2):190-199. doi: 10.1016/j.annonc.2023.09.3108. Epub 2023 Oct 21. Ann Oncol. 2024. PMID: 37872020 Clinical Trial.
-
Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma.Eur Urol. 2014 Nov;66(5):874-80. doi: 10.1016/j.eururo.2014.01.035. Epub 2014 Feb 7. Eur Urol. 2014. PMID: 24560330 Free PMC article. Clinical Trial.
-
Efficacy and safety of neoadjuvant therapy with tislelizumab plus axitinib for nonmetastatic renal cell carcinoma with inferior vena cava tumor thrombus: a retrospective study.Sci Rep. 2025 Jan 17;15(1):2248. doi: 10.1038/s41598-025-86712-6. Sci Rep. 2025. PMID: 39833373 Free PMC article.
-
Avelumab and axitinib combination therapy for the treatment of advanced renal cell carcinoma.Future Oncol. 2020 Dec;16(36):3021-3034. doi: 10.2217/fon-2020-0586. Epub 2020 Aug 28. Future Oncol. 2020. PMID: 32856478 Review.
-
Avelumab and axitinib in the treatment of renal cell carcinoma: safety and efficacy.Expert Rev Anticancer Ther. 2020 May;20(5):343-354. doi: 10.1080/14737140.2020.1756780. Epub 2020 May 7. Expert Rev Anticancer Ther. 2020. PMID: 32293937 Review.
Cited by
-
A Review of Neoadjuvant Therapy for Localized and Locally Advanced Renal Cell Carcinoma.Cancers (Basel). 2025 Jan 19;17(2):312. doi: 10.3390/cancers17020312. Cancers (Basel). 2025. PMID: 39858094 Free PMC article. Review.
-
Role of Systemic Therapy in Localized Renal Cell Carcinoma: Where Do We Stand and Where Are We Heading?Cancers (Basel). 2025 May 14;17(10):1656. doi: 10.3390/cancers17101656. Cancers (Basel). 2025. PMID: 40427152 Free PMC article. Review.
-
Role of Neoadjuvant Immunotherapy in Genitourinary Malignancies.Cancers (Basel). 2024 Dec 10;16(24):4127. doi: 10.3390/cancers16244127. Cancers (Basel). 2024. PMID: 39766027 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
