RNase P: Beyond Precursor tRNA Processing
- PMID: 38862431
- PMCID: PMC12016569
- DOI: 10.1093/gpbjnl/qzae016
RNase P: Beyond Precursor tRNA Processing
Abstract
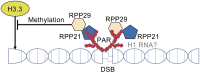
Ribonuclease P (RNase P) was first described in the 1970's as an endoribonuclease acting in the maturation of precursor transfer RNAs (tRNAs). More recent studies, however, have uncovered non-canonical roles for RNase P and its components. Here, we review the recent progress of its involvement in chromatin assembly, DNA damage response, and maintenance of genome stability with implications in tumorigenesis. The possibility of RNase P as a therapeutic target in cancer is also discussed.
Keywords: Chromatin assembly; DNA damage response; Genome stability; RNase P; Tumorigenesis.
© The Author(s) 2024. Published by Oxford University Press and Science Press on behalf of the Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation and Genetics Society of China.
Conflict of interest statement
The authors have declared no competing interests.
Figures
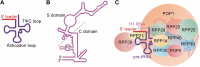
Similar articles
-
Multiple structural flavors of RNase P in precursor tRNA processing.Wiley Interdiscip Rev RNA. 2024 Mar-Apr;15(2):e1835. doi: 10.1002/wrna.1835. Wiley Interdiscip Rev RNA. 2024. PMID: 38479802 Review.
-
Coordination of transcription and processing of tRNA.FEBS J. 2022 Jul;289(13):3630-3641. doi: 10.1111/febs.15904. Epub 2021 May 11. FEBS J. 2022. PMID: 33929081 Review.
-
Structural basis for human mitochondrial tRNA maturation.Nat Commun. 2024 Jun 1;15(1):4683. doi: 10.1038/s41467-024-49132-0. Nat Commun. 2024. PMID: 38824131 Free PMC article.
-
Characterization of conserved sequence elements in eukaryotic RNase P RNA reveals roles in holoenzyme assembly and tRNA processing.RNA. 2005 Jun;11(6):885-96. doi: 10.1261/rna.7282205. Epub 2005 May 4. RNA. 2005. PMID: 15872187 Free PMC article.
-
The rph-1-Encoded Truncated RNase PH Protein Inhibits RNase P Maturation of Pre-tRNAs with Short Leader Sequences in the Absence of RppH.J Bacteriol. 2017 Oct 17;199(22):e00301-17. doi: 10.1128/JB.00301-17. Print 2017 Nov 15. J Bacteriol. 2017. PMID: 28808133 Free PMC article.
Cited by
-
RNA Polymerase III-Transcribed RNAs in Health and Disease: Mechanisms, Dysfunction, and Future Directions.Int J Mol Sci. 2025 Jun 18;26(12):5852. doi: 10.3390/ijms26125852. Int J Mol Sci. 2025. PMID: 40565315 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

