Glucose deprivation triggers DCAF1-mediated inactivation of Rheb-mTORC1 and promotes cancer cell survival
- PMID: 38862475
- PMCID: PMC11166663
- DOI: 10.1038/s41419-024-06808-1
Glucose deprivation triggers DCAF1-mediated inactivation of Rheb-mTORC1 and promotes cancer cell survival
Abstract
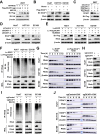
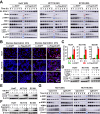
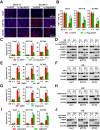
Low glucose is a common microenvironment for rapidly growing solid tumors, which has developed multiple approaches to survive under glucose deprivation. However, the specific regulatory mechanism remains largely elusive. In this study, we demonstrate that glucose deprivation, while not amino acid or serum starvation, transactivates the expression of DCAF1. This enhances the K48-linked polyubiquitination and proteasome-dependent degradation of Rheb, inhibits mTORC1 activity, induces autophagy, and facilitates cancer cell survival under glucose deprivation conditions. This study identified DCAF1 as a new cellular glucose sensor and uncovered new insights into mechanism of DCAF1-mediated inactivation of Rheb-mTORC1 pathway for promoting cancer cell survival in response to glucose deprivation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Lysosomal EGFR acts as a Rheb-GEF independent of its kinase activity to activate mTORC1.Cell Res. 2025 Jul;35(7):497-509. doi: 10.1038/s41422-025-01110-x. Epub 2025 Apr 21. Cell Res. 2025. PMID: 40259053 Free PMC article.
-
Ubiquitination of Rheb governs growth factor-induced mTORC1 activation.Cell Res. 2019 Feb;29(2):136-150. doi: 10.1038/s41422-018-0120-9. Epub 2018 Dec 4. Cell Res. 2019. PMID: 30514904 Free PMC article.
-
Dynamin-dependent amino acid endocytosis activates mechanistic target of rapamycin complex 1 (mTORC1).J Biol Chem. 2017 Nov 3;292(44):18052-18061. doi: 10.1074/jbc.M117.776443. Epub 2017 Aug 14. J Biol Chem. 2017. PMID: 28808055 Free PMC article.
-
Not Just PA28γ: What We Know About the Role of PA28αβ in Carcinogenesis.Biomolecules. 2025 Jun 16;15(6):880. doi: 10.3390/biom15060880. Biomolecules. 2025. PMID: 40563520 Free PMC article. Review.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
Cited by
-
RHEB neddylation by the UBE2F-SAG axis enhances mTORC1 activity and aggravates liver tumorigenesis.EMBO J. 2025 Feb;44(4):1185-1219. doi: 10.1038/s44318-024-00353-5. Epub 2025 Jan 6. EMBO J. 2025. PMID: 39762645 Free PMC article.
-
Prognostic Protein Biomarker Screening for Thyroid Carcinoma Based on Cancer Proteomics Profiles.Biomedicines. 2024 Sep 10;12(9):2066. doi: 10.3390/biomedicines12092066. Biomedicines. 2024. PMID: 39335579 Free PMC article.
-
Post-translational modifications orchestrate mTOR-driven cell death in cardiovascular disease.Front Cardiovasc Med. 2025 Jul 15;12:1620669. doi: 10.3389/fcvm.2025.1620669. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40734978 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

