Transcranial focused ultrasound to V5 enhances human visual motion brain-computer interface by modulating feature-based attention
- PMID: 38862476
- PMCID: PMC11167030
- DOI: 10.1038/s41467-024-48576-8
Transcranial focused ultrasound to V5 enhances human visual motion brain-computer interface by modulating feature-based attention
Abstract
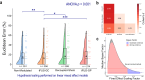
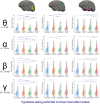
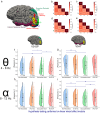
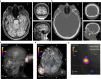
A brain-computer interface (BCI) enables users to control devices with their minds. Despite advancements, non-invasive BCIs still exhibit high error rates, prompting investigation into the potential reduction through concurrent targeted neuromodulation. Transcranial focused ultrasound (tFUS) is an emerging non-invasive neuromodulation technology with high spatiotemporal precision. This study examines whether tFUS neuromodulation can improve BCI outcomes, and explores the underlying mechanism of action using high-density electroencephalography (EEG) source imaging (ESI). As a result, V5-targeted tFUS significantly reduced the error in a BCI speller task. Source analyses revealed a significantly increase in theta and alpha activities in the tFUS condition at both V5 and downstream in the dorsal visual processing pathway. Correlation analysis indicated that the connection within the dorsal processing pathway was preserved during tFUS stimulation, while the ventral connection was weakened. These findings suggest that V5-targeted tFUS enhances feature-based attention to visual motion.
© 2024. The Author(s).
Conflict of interest statement
B.H. and K.Y. are co-inventors of a pending US patent application (Applicant: Carnegie Mellon University; Inventors: Bin He and Kai Yu; Application No: 18/553,901; Status: Pending; Specific aspect: tFUS and electrophysiological source imaging). J.K. and C.L. have no competing interests to declare.
Figures

Update of
-
Transcranial Focused Ultrasound to V5 Enhances Human Visual Motion Brain-Computer Interface by Modulating Feature-Based Attention.bioRxiv [Preprint]. 2023 Sep 5:2023.09.04.556252. doi: 10.1101/2023.09.04.556252. bioRxiv. 2023. Update in: Nat Commun. 2024 Jun 11;15(1):4382. doi: 10.1038/s41467-024-48576-8. PMID: 37732253 Free PMC article. Updated. Preprint.
Similar articles
-
Transcranial Focused Ultrasound to V5 Enhances Human Visual Motion Brain-Computer Interface by Modulating Feature-Based Attention.bioRxiv [Preprint]. 2023 Sep 5:2023.09.04.556252. doi: 10.1101/2023.09.04.556252. bioRxiv. 2023. Update in: Nat Commun. 2024 Jun 11;15(1):4382. doi: 10.1038/s41467-024-48576-8. PMID: 37732253 Free PMC article. Updated. Preprint.
-
Transcranial Focused Ultrasound Neuromodulation of Voluntary Movement-Related Cortical Activity in Humans.IEEE Trans Biomed Eng. 2021 Jun;68(6):1923-1931. doi: 10.1109/TBME.2020.3030892. Epub 2021 May 21. IEEE Trans Biomed Eng. 2021. PMID: 33055021 Free PMC article.
-
Transcranial Focused Ultrasound Enhances Sensory Discrimination Capability through Somatosensory Cortical Excitation.Ultrasound Med Biol. 2021 May;47(5):1356-1366. doi: 10.1016/j.ultrasmedbio.2021.01.025. Epub 2021 Feb 20. Ultrasound Med Biol. 2021. PMID: 33622622 Free PMC article.
-
Advances in transcranial focused ultrasound neuromodulation for mental disorders.Prog Neuropsychopharmacol Biol Psychiatry. 2025 Jan 10;136:111244. doi: 10.1016/j.pnpbp.2024.111244. Epub 2025 Jan 3. Prog Neuropsychopharmacol Biol Psychiatry. 2025. PMID: 39756638 Review.
-
A review of functional neuromodulation in humans using low-intensity transcranial focused ultrasound.Biomed Eng Lett. 2024 Mar 2;14(3):407-438. doi: 10.1007/s13534-024-00369-0. eCollection 2024 May. Biomed Eng Lett. 2024. PMID: 38645585 Free PMC article. Review.
Cited by
-
The future of transcranial ultrasound as a precision brain interface.PLoS Biol. 2024 Oct 29;22(10):e3002884. doi: 10.1371/journal.pbio.3002884. eCollection 2024 Oct. PLoS Biol. 2024. PMID: 39471185 Free PMC article.
-
Examination of the interaction of parameters for low-intensity focused ultrasound of the human motor cortex.Brain Stimul. 2024 Nov-Dec;17(6):1293-1306. doi: 10.1016/j.brs.2024.11.005. Epub 2024 Nov 21. Brain Stimul. 2024. PMID: 39577741 Free PMC article.
-
Achieving High-Performance Transcranial Ultrasound Transmission Through Mie and Fano Resonance in Flexible Metamaterials.Adv Sci (Weinh). 2025 May;12(19):e2500170. doi: 10.1002/advs.202500170. Epub 2025 Mar 26. Adv Sci (Weinh). 2025. PMID: 40135785 Free PMC article.
-
Non-Invasive Brain-Computer Interfaces: State of the Art and Trends.IEEE Rev Biomed Eng. 2025;18:26-49. doi: 10.1109/RBME.2024.3449790. Epub 2025 Jan 28. IEEE Rev Biomed Eng. 2025. PMID: 39186407 Free PMC article. Review.
-
Label free, capillary-scale blood flow mapping in vivo reveals that low-intensity focused ultrasound evokes persistent dilation in cortical microvasculature.Commun Biol. 2025 Jan 6;8(1):12. doi: 10.1038/s42003-024-07356-2. Commun Biol. 2025. PMID: 39762513 Free PMC article.
References
-
- Harvey EN. The effect of high frequency sound waves on heart muscle and other irritable tissues. Am. J. Physiol. Leg. Content. 1929;91:284–290. doi: 10.1152/ajplegacy.1929.91.1.284. - DOI
MeSH terms
Grants and funding
- U18 EB029354/EB/NIBIB NIH HHS/United States
- DGE2140739/National Science Foundation (NSF)
- R01NS124564, RF1NS131069, R01NS096761, and NS127849/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 AT009263/AT/NCCIH NIH HHS/United States
- R01 NS124564/NS/NINDS NIH HHS/United States
- T32 EB029365/EB/NIBIB NIH HHS/United States
- R01AT009263/U.S. Department of Health & Human Services | NIH | National Center for Complementary and Integrative Health (NCCIH)
- RF1 NS131069/NS/NINDS NIH HHS/United States
- T32EB029365/U.S. Department of Health & Human Services | NIH | National Institute of Biomedical Imaging and Bioengineering (NIBIB)
- R01 NS127849/NS/NINDS NIH HHS/United States
- R01 NS096761/NS/NINDS NIH HHS/United States
- U18EB029354, T32EB029365/U.S. Department of Health & Human Services | NIH | National Institute of Biomedical Imaging and Bioengineering (NIBIB)
LinkOut - more resources
Full Text Sources
Research Materials

