Microdroplets initiate organic-inorganic interactions and mass transfer in thermal hydrous geosystems
- PMID: 38862499
- PMCID: PMC11167059
- DOI: 10.1038/s41467-024-49293-y
Microdroplets initiate organic-inorganic interactions and mass transfer in thermal hydrous geosystems
Abstract
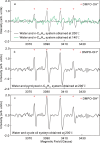
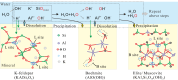
Organic-inorganic interactions regulate the dynamics of hydrocarbons, water, minerals, CO2, and H2 in thermal rocks, yet their initiation remains debated. To address this, we conducted isotope-tagged and in-situ visual thermal experiments. Isotope-tagged studies revealed extensive H/O transfers in hydrous n-C20H42-H2O-feldspar systems. Visual experiments observed water microdroplets forming at 150-165 °C in oil phases near the water-oil interface without surfactants, persisting until complete miscibility above 350 °C. Electron paramagnetic resonance (EPR) detected hydroxyl free radicals concurrent with microdroplet formation. Here we propose a two-fold mechanism: water-derived and n-C20H42-derived free radicals drive interactions with organic species, while water-derived and mineral-derived ions trigger mineral interactions. These processes, facilitated by microdroplets and bulk water, blur boundaries between organic and inorganic species, enabling extensive interactions and mass transfer. Our findings redefine microscopic interplays between organic and inorganic components, offering insights into diagenetic and hydrous-metamorphic processes, and mass transfer cycles in deep basins and subduction zones.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Tao R, et al. Formation of abiotic hydrocarbon from reduction of carbonate in subduction zones: constraints from petrological observation and experimental simulation. Geochim. Cosmochim. Acta. 2018;239:390–408. doi: 10.1016/j.gca.2018.08.008. - DOI
-
- Yuan G, et al. Coupled mineral alteration and oil degradation in thermal oil-water-feldspar systems and implications for organic-inorganic interactions in hydrocarbon reservoirs. Geochim. Cosmochim. Acta. 2019;248:61–87. doi: 10.1016/j.gca.2019.01.001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

