ER-mitochondria association negatively affects wound healing by regulating NLRP3 activation
- PMID: 38862500
- PMCID: PMC11167056
- DOI: 10.1038/s41419-024-06765-9
ER-mitochondria association negatively affects wound healing by regulating NLRP3 activation
Abstract
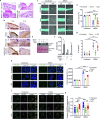
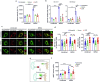
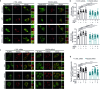
Methicillin-resistant Staphylococcus aureus (MRSA) is the most common causative agent of acute bacterial skin and skin-structure infections (ABSSSI), one of the major challenges to the health system worldwide. Although the use of antibiotics as the first line of intervention for MRSA-infected wounds is recommended, important side effects could occur, including cytotoxicity or immune dysregulation, thus affecting the repair process. Here, we show that the oxazolidinone antibiotic linezolid (LZD) impairs wound healing by aberrantly increasing interleukin 1 β (IL-1β) production in keratinocytes. Mechanistically, LZD triggers a reactive oxygen species (ROS)-independent mitochondrial damage that culminates in increased tethering between the endoplasmic reticulum (ER) and mitochondria, which in turn activates the NLR family pyrin domain-containing 3 (NLRP3) inflammasome complex by promoting its assembly to the mitochondrial surface. Downregulation of ER-mitochondria contact formation is sufficient to inhibit the LZD-driven NLRP3 inflammasome activation and IL-1β production, restoring wound closure. These results identify the ER-mitochondria association as a key factor for NLRP3 activation and reveal a new mechanism in the regulation of the wound healing process that might be clinically relevant.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Giacobbe DR, Dettori S, Corcione S, Vena A, Sepulcri C, Maraolo AE, et al. Emerging treatment options for acute bacterial skin and skin structure infections and bloodstream infections caused by staphylococcus aureus: a comprehensive review of the evidence. Infect Drug Resistance. 2022;15:2137–57. doi: 10.2147/IDR.S318322. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

