Viviparity imparts a macroevolutionary signature of ecological opportunity in the body size of female Liolaemus lizards
- PMID: 38862522
- PMCID: PMC11167029
- DOI: 10.1038/s41467-024-49464-x
Viviparity imparts a macroevolutionary signature of ecological opportunity in the body size of female Liolaemus lizards
Abstract
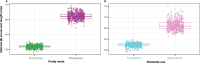
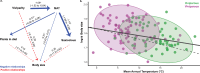
Viviparity evolved ~115 times across squamate reptiles, facilitating the colonization of cold habitats, where oviparous species are scarce or absent. Whether the ecological opportunity furnished by such colonization reconfigures phenotypic diversity and accelerates evolution is unclear. We investigated the association between viviparity and patterns and rates of body size evolution in female Liolaemus lizards, the most species-rich tetrapod genus from temperate regions. Here, we discover that viviparous species evolve ~20% larger optimal body sizes than their oviparous relatives, but exhibit similar rates of body size evolution. Through a causal modeling approach, we find that viviparity indirectly influences body size evolution through shifts in thermal environment. Accordingly, the colonization of cold habitats favors larger body sizes in viviparous species, reconfiguring body size diversity in Liolaemus. The catalyzing influence of viviparity on phenotypic evolution arises because it unlocks access to otherwise inaccessible sources of ecological opportunity, an outcome potentially repeated across the tree of life.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Differential reproductive investment in co-occurring oviparous and viviparous common lizards (Zootoca vivipara) and implications for life-history trade-offs with viviparity.Oecologia. 2019 May;190(1):85-98. doi: 10.1007/s00442-019-04398-w. Epub 2019 May 6. Oecologia. 2019. PMID: 31062164 Free PMC article.
-
Placental specializations in lecithotrophic viviparous squamate reptiles.J Exp Zool B Mol Dev Evol. 2015 Sep;324(6):549-61. doi: 10.1002/jez.b.22632. Epub 2015 Jun 7. J Exp Zool B Mol Dev Evol. 2015. PMID: 26055953
-
Exceptional parallelisms characterize the evolutionary transition to live birth in phrynosomatid lizards.Nat Commun. 2022 May 24;13(1):2881. doi: 10.1038/s41467-022-30535-w. Nat Commun. 2022. PMID: 35610218 Free PMC article.
-
Ancestral state reconstructions require biological evidence to test evolutionary hypotheses: A case study examining the evolution of reproductive mode in squamate reptiles.J Exp Zool B Mol Dev Evol. 2015 Sep;324(6):493-503. doi: 10.1002/jez.b.22614. Epub 2015 Mar 2. J Exp Zool B Mol Dev Evol. 2015. PMID: 25732809 Review.
-
A review of the evolution of viviparity in squamate reptiles: the past, present and future role of molecular biology and genomics.J Comp Physiol B. 2011 Jul;181(5):575-94. doi: 10.1007/s00360-011-0584-0. Epub 2011 May 15. J Comp Physiol B. 2011. PMID: 21573966 Review.
Cited by
-
Opportunity begets opportunity to drive macroevolutionary dynamics of a diverse lizard radiation.Evol Lett. 2024 Jun 3;8(5):623-637. doi: 10.1093/evlett/qrae022. eCollection 2024 Sep. Evol Lett. 2024. PMID: 39328284 Free PMC article.
-
The micro-niche explains allotopy and syntopy in South American Liolaemus (Iguania: Liolaemidae) lizards.PeerJ. 2025 Feb 17;13:e18979. doi: 10.7717/peerj.18979. eCollection 2025. PeerJ. 2025. PMID: 39981051 Free PMC article.
References
-
- Shine R. Evolution of an evolutionary hypothesis: a history of changing ideas about the adaptive significance of viviparity in reptiles. J. Herpetol. 2014;48:147–161. doi: 10.1670/13-075. - DOI
-
- Beuchat CA. Reproductive influences on the thermoregulatory behavior of a live-bearing lizard. Copeia. 1986;4:971–979. doi: 10.2307/1445294. - DOI
-
- Beuchat CA. Temperature effects during gestation in a viviparous lizard. J. Therm. Biol. 1988;13:135–142. doi: 10.1016/0306-4565(88)90024-1. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

