Combined oral low-dose cyclophosphamide endocrine therapy may improve clinical response among patients with metastatic breast cancer via Tregs in TLSs
- PMID: 38862586
- PMCID: PMC11166640
- DOI: 10.1038/s41598-024-64042-3
Combined oral low-dose cyclophosphamide endocrine therapy may improve clinical response among patients with metastatic breast cancer via Tregs in TLSs
Abstract
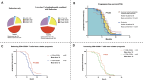
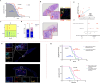
Despite limited research on refractory and/or endocrine therapy failure in elderly metastatic breast cancer (MBC) patients, a prior study showed that low-dose oral cyclophosphamide (CY) can improve the overall survival rate of MBC patients, possibly through the immunoregulation of regulatory T cells (Tregs). We preliminarily investigated the combination of endocrine therapy (ET) with oral low-dose CY as salvage therapy in elderly patients via peripheral blood regulatory T-cell analyses. In addition, we evaluated the associations of tumor tertiary lymphoid structures (TLSs) with therapeutic outcomes. HR+/HER2- advanced breast cancer patients who received low-dose CY combined with ET or ET only from April 2015 to August 2021 were enrolled in this retrospective study. The primary outcome was the clinical control rate (CCR), and the secondary outcome was progression-free survival (PFS). Circulating T lymphocyte subpopulations represented by Tregs were monitored during treatment by flow cytometry methods. TLSs wereconfirmed by hematoxylin-eosin staining of pretreatment specimens, and CD3, CD4, and Foxp3 were detected using Opal multicolor immunofluorescence. A total of 85 patients who received CY + ET and 50 patients who received ET only were enrolled, the percentage of patients who received CCR was 73% (62/85) vs. 70% (45/50), and the objective response rate (ORR) was 28% (24/85) vs. 24% (12/50). No deaths occurred during the study period. The mean PFS time was 13 vs. 11 months (P = 0.03). In the CY + ET group, decreases in CD4+/CD25+/Foxp3+ T cells (P < 0.001) were favorable for both clinical control and prolonged PFS (P < 0.001). Compared with patients without TLSs, those with TLSs were more likely to have better clinical control and PFS (mean time = 6 months), and a greater number of Treg cells during TLS pretreatment correlated with longer PFS (P = 0.043). Oral low-dose CY combined with standard ET exerts immunological effects by decreasing Treg levels to achieve improved clinical responses. Moreover, patients with TLSs might benefit more from such therapy than those without TLSs, and a high Treg cell count in TLSs before treatment predicts better therapeutic efficacy.
Keywords: Cyclophosphamide; Low dose; Metastatic breast cancer; Regulatory T cell; Tertiary lymphoid structures; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Rose C, Vtoraya O, Pluzanska A, Davidson N, Gershanovich M, Thomas R, et al. An open randomised trial of second-line endocrine therapy in advanced breast cancer comparison of the aromatase inhibitors letrozole and anastrozole. Eur. J. Cancer. 2003;39:2318–2327. doi: 10.1016/S0959-8049(03)00630-0. - DOI - PubMed
-
- Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J. Clin. Oncol. 2008;26:1664–1670. doi: 10.1200/JCO.2007.13.5822. - DOI - PubMed
-
- Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J. Clin. Oncol. 2010;28:4594–4600. doi: 10.1200/JCO.2010.28.8415. - DOI - PubMed
-
- Thurlimann B, Robertson JF, Nabholtz JM, Buzdar A, Bonneterre J, Arimidex Study Group Efficacy of tamoxifen following anastrozole (‘Arimidex’) compared with anastrozole following tamoxifen as first-line treatment for advanced breast cancer in postmenopausal women. Eur. J. Cancer. 2003;39:2310–2317. doi: 10.1016/S0959-8049(03)00602-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

