Multi-omics analysis reveals the dynamic interplay between Vero host chromatin structure and function during vaccinia virus infection
- PMID: 38862613
- PMCID: PMC11166932
- DOI: 10.1038/s42003-024-06389-x
Multi-omics analysis reveals the dynamic interplay between Vero host chromatin structure and function during vaccinia virus infection
Abstract
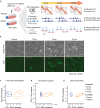
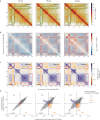
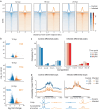
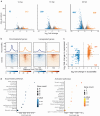
The genome folds into complex configurations and structures thought to profoundly impact its function. The intricacies of this dynamic structure-function relationship are not well understood particularly in the context of viral infection. To unravel this interplay, here we provide a comprehensive investigation of simultaneous host chromatin structural (via Hi-C and ATAC-seq) and functional changes (via RNA-seq) in response to vaccinia virus infection. Over time, infection significantly impacts global and local chromatin structure by increasing long-range intra-chromosomal interactions and B compartmentalization and by decreasing chromatin accessibility and inter-chromosomal interactions. Local accessibility changes are independent of broad-scale chromatin compartment exchange (~12% of the genome), underscoring potential independent mechanisms for global and local chromatin reorganization. While infection structurally condenses the host genome, there is nearly equal bidirectional differential gene expression. Despite global weakening of intra-TAD interactions, functional changes including downregulated immunity genes are associated with alterations in local accessibility and loop domain restructuring. Therefore, chromatin accessibility and local structure profiling provide impactful predictions for host responses and may improve development of efficacious anti-viral counter measures including the optimization of vaccine design.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
ATAC-seq for Characterizing Host and Pathogen Genome Accessibility During Virus Infection.Methods Mol Biol. 2025;2866:111-122. doi: 10.1007/978-1-0716-4192-7_7. Methods Mol Biol. 2025. PMID: 39546200
-
The Host Restriction Factor Interferon-Inducible Transmembrane Protein 3 Inhibits Vaccinia Virus Infection.Front Immunol. 2018 Feb 16;9:228. doi: 10.3389/fimmu.2018.00228. eCollection 2018. Front Immunol. 2018. PMID: 29503647 Free PMC article.
-
Sequence and phylogenetic analysis of host-range (E3L, K3L, and C7L) and structural protein (B5R) genes of buffalopox virus isolates from buffalo, cattle, and human in India.Virus Genes. 2012 Dec;45(3):488-98. doi: 10.1007/s11262-012-0788-8. Epub 2012 Aug 8. Virus Genes. 2012. PMID: 22872567
-
Computational Integration of HSV-1 Multi-omics Data.Methods Mol Biol. 2023;2610:31-48. doi: 10.1007/978-1-0716-2895-9_3. Methods Mol Biol. 2023. PMID: 36534279 Review.
-
Vaccinia Virus Protein C6: A Multifunctional Interferon Antagonist.Adv Exp Med Biol. 2018;1052:1-7. doi: 10.1007/978-981-10-7572-8_1. Adv Exp Med Biol. 2018. PMID: 29785476 Review.
Cited by
-
From 2D to 4D: a Containerized Workflow and Browser to Explore Dynamic Chromatin Architecture.bioRxiv [Preprint]. 2025 Jul 18:2025.07.13.664622. doi: 10.1101/2025.07.13.664622. bioRxiv. 2025. PMID: 40791469 Free PMC article. Preprint.
-
Applying 3D Genome Technology in Virology Research.Methods Mol Biol. 2025;2940:43-61. doi: 10.1007/978-1-0716-4615-1_6. Methods Mol Biol. 2025. PMID: 40515901
-
Elucidating the Mechanism of VVTT Infection Through Machine Learning and Transcriptome Analysis.Int J Mol Sci. 2025 Jan 30;26(3):1203. doi: 10.3390/ijms26031203. Int J Mol Sci. 2025. PMID: 39940969 Free PMC article.
-
Best practices for methylome characterization in novel species: a case study in the microalgae Microchloropsis.Commun Biol. 2025 Apr 23;8(1):648. doi: 10.1038/s42003-025-08027-6. Commun Biol. 2025. PMID: 40269108 Free PMC article.
-
Chromatin structure and gene transcription of recombinant p53 adenovirus vector within host.Front Mol Biosci. 2025 Feb 28;12:1562357. doi: 10.3389/fmolb.2025.1562357. eCollection 2025. Front Mol Biosci. 2025. PMID: 40092712 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

