Cumulative incidence and risk factors for medication-related osteonecrosis of the jaw during long-term prostate cancer management
- PMID: 38862617
- PMCID: PMC11167048
- DOI: 10.1038/s41598-024-64440-7
Cumulative incidence and risk factors for medication-related osteonecrosis of the jaw during long-term prostate cancer management
Abstract
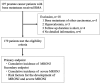
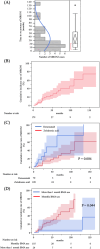
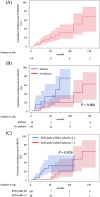
Bone-modifying agents (BMA) are extensively used in treating patients with prostate cancer with bone metastases. However, this increases the risk of medication-related osteonecrosis of the jaw (MRONJ). The safety of long-term BMA administration in clinical practice remains unclear. We aimed to determine the cumulative incidence and risk factors of MRONJ. One hundred and seventy-nine patients with prostate cancer with bone metastases treated with BMA at our institution since 2008 were included in this study. Twenty-seven patients (15%) had MRONJ during the follow-up period (median, 19 months; interquartile range, 9-43 months). The 2-year, 5-year, and 10-year cumulative MRONJ incidence rates were 18%, 27%, and 61%, respectively. Multivariate analysis identified denosumab use as a risk factor for MRONJ, compared with zoledronic acid use (HR 4.64, 95% CI 1.93-11.1). Additionally, BMA use at longer than one-month intervals was associated with a lower risk of MRONJ (HR 0.08, 95% CI 0.01-0.64). Furthermore, six or more bone metastases (HR 3.65, 95% CI 1.13-11.7) and diabetes mellitus (HR 5.07, 95% CI 1.68-15.2) were risk factors for stage 2 or more severe MRONJ. MRONJ should be considered during long-term BMA administration in prostate cancer patients with bone metastases.
Keywords: Denosumab; Jaw; Osteonecrosis; Prostate cancer; Zoledronic acid.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- National Comprehensive Cancer Network (2019) NCCN clinical practice guidelines in oncology prostate cancer version 4.
-
- Kishimoto H, Kurita H. The number of patients with bisphosphonate-related osteonecrosis of the jaws still increases significantly in Japan e based on the data from annual survey of the oral diseases by JAOMS. Jpn J. Oral Maxillofac. Surg. 2022;68:161–163. doi: 10.5794/jjoms.68.161. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

