Electrodiagnostic tests of the visual pathway and applications in neuro-ophthalmology
- PMID: 38862643
- PMCID: PMC11306601
- DOI: 10.1038/s41433-024-03154-6
Electrodiagnostic tests of the visual pathway and applications in neuro-ophthalmology
Abstract
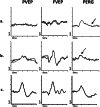
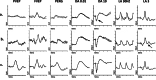
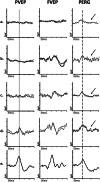
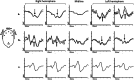
This article describes the main visual electrodiagnostic tests relevant to neuro-ophthalmology practice, including the visual evoked potential (VEP), and the full-field, pattern and multifocal electroretinograms (ffERG; PERG; mfERG). The principles of electrophysiological interpretation are illustrated with reference to acquired and inherited optic neuropathies, and retinal disorders that may masquerade as optic neuropathy, including ffERG and PERG findings in cone and macular dystrophies, paraneoplastic and vascular retinopathies. Complementary VEP and PERG recordings are illustrated in demyelinating, ischaemic, nutritional (B12), and toxic (mercury, cobalt, and ethambutol-related) optic neuropathies and inherited disorders affecting mitochondrial function such as Leber hereditary optic neuropathy and dominant optic atrophy. The value of comprehensive electrophysiological phenotyping in syndromic diseases is highlighted in cases of SSBP1-related disease and ROSAH (Retinal dystrophy, Optic nerve oedema, Splenomegaly, Anhidrosis and Headache). The review highlights the value of different electrophysiological techniques, for the purposes of differential diagnosis and objective functional phenotyping.
摘要: 本文介绍了与神经眼科临床相关的主要视觉电生理诊断试验, 包括视觉诱发电位(VEP), 以及全视野ERG, 图形ERG以及多焦ERG(ffERG;PERG;mfERG)。电生理学检查的基本原则以及对获得性和遗传性视神经病变, 以及与视神经病变需要鉴别的其它视网膜病变, 如视锥细胞与黄斑营养不良、副肿瘤和血管视网膜病变等。VEP和PERG在脱髓鞘、缺血、营养性(B12)和毒性(汞、钴和乙胺丁醇相关)视神经病变以及影响线粒体功能的遗传性疾病的诊断中起到了重要作用, 如Leber遗传性视神经病变和显性视神经萎缩。在SSBP1相关疾病和ROSAH(视网膜营养不良、视神经水肿、脾肿大、多汗和头痛)的病例中, 电生理改变在全身性疾病中的诊断性价值尤为突出。本综述强调了不同的电生理技术在鉴别诊断和客观的视网膜功能以及疾病表型方面的价值。.
© 2024. The Author(s).
Conflict of interest statement
NJ: Consultancy fees received from Chiesi, AC, MMN, AGR: None
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

