Activation of the cell wall integrity pathway negatively regulates TORC2-Ypk1/2 signaling through blocking eisosome disassembly in Saccharomyces cerevisiae
- PMID: 38862688
- PMCID: PMC11166964
- DOI: 10.1038/s42003-024-06411-2
Activation of the cell wall integrity pathway negatively regulates TORC2-Ypk1/2 signaling through blocking eisosome disassembly in Saccharomyces cerevisiae
Abstract
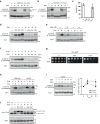
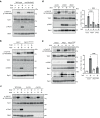
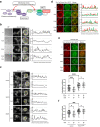
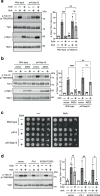
The target of rapamycin complex 2 (TORC2) signaling is associated with plasma membrane (PM) integrity. In Saccharomyces cerevisiae, TORC2-Ypk1/2 signaling controls sphingolipid biosynthesis, and Ypk1/2 phosphorylation by TORC2 under PM stress conditions is increased in a Slm1/2-dependent manner, under which Slm1 is known to be released from an eisosome, a furrow-like invagination PM structure. However, it remains unsolved how the activation machinery of TORC2-Ypk1/2 signaling is regulated. Here we show that edelfosine, a synthetic lysophospholipid analog, inhibits the activation of TORC2-Ypk1/2 signaling, and the cell wall integrity (CWI) pathway is involved in this inhibitory effect. The activation of CWI pathway blocked the eisosome disassembly promoted by PM stress and the release of Slm1 from eisosomes. Constitutive activation of TORC2-Ypk1/2 signaling exhibited increased sensitivity to cell wall stress. We propose that the CWI pathway negatively regulates the TORC2-Ypk1/2 signaling, which is involved in the regulatory mechanism to ensure the proper stress response to cell wall damage.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The Stress-Sensing TORC2 Complex Activates Yeast AGC-Family Protein Kinase Ypk1 at Multiple Novel Sites.Genetics. 2017 Sep;207(1):179-195. doi: 10.1534/genetics.117.1124. Epub 2017 Jul 24. Genetics. 2017. PMID: 28739659 Free PMC article.
-
The TORC2-Dependent Signaling Network in the Yeast Saccharomyces cerevisiae.Biomolecules. 2017 Sep 5;7(3):66. doi: 10.3390/biom7030066. Biomolecules. 2017. PMID: 28872598 Free PMC article. Review.
-
Rab5 GTPases are required for optimal TORC2 function.J Cell Biol. 2019 Mar 4;218(3):961-976. doi: 10.1083/jcb.201807154. Epub 2018 Dec 21. J Cell Biol. 2019. PMID: 30578283 Free PMC article.
-
Target of rapamycin complex 2-dependent phosphorylation of the coat protein Pan1 by Akl1 controls endocytosis dynamics in Saccharomyces cerevisiae.J Biol Chem. 2018 Aug 3;293(31):12043-12053. doi: 10.1074/jbc.RA117.001615. Epub 2018 Jun 12. J Biol Chem. 2018. PMID: 29895620 Free PMC article.
-
Regulation of TORC2 function and localization by Rab5 GTPases in Saccharomyces cerevisiae.Cell Cycle. 2019 May;18(10):1084-1094. doi: 10.1080/15384101.2019.1616999. Epub 2019 May 15. Cell Cycle. 2019. PMID: 31068077 Free PMC article. Review.
Cited by
-
Possible regulatory network and associated pathways governing the expression of ADH2 in Saccharomyces cerevisiae.Curr Genet. 2025 Aug 18;71(1):15. doi: 10.1007/s00294-025-01321-0. Curr Genet. 2025. PMID: 40824391 Review.
-
Enhancing Chitin Production as a Fermentation Byproduct through a Genetic Toolbox That Activates the Cell Wall Integrity Response.ACS Synth Biol. 2025 Jan 17;14(1):113-128. doi: 10.1021/acssynbio.4c00436. Epub 2025 Jan 6. ACS Synth Biol. 2025. PMID: 39757911 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

