Relationship between pruritus and sleep in participants with primary biliary cholangitis in the Phase 2b GLIMMER trial
- PMID: 38862718
- PMCID: PMC11166618
- DOI: 10.1186/s41687-024-00722-y
Relationship between pruritus and sleep in participants with primary biliary cholangitis in the Phase 2b GLIMMER trial
Abstract
Background: Cholestatic pruritus and fatigue are debilitating conditions associated with primary biliary cholangitis (PBC) and can significantly impact patients' quality of life. Pruritus in PBC often worsens at night and patients frequently report sleep disturbance, which contributes to cognitive symptoms and fatigue. Linerixibat is an ileal bile acid transporter inhibitor in clinical development for the treatment of pruritus associated with PBC and was recently assessed versus placebo in the Phase 2b GLIMMER trial. This post-hoc analysis assesses the relationship between pruritus severity and sleep disturbance in participants of GLIMMER regardless of treatment group.
Methods: GLIMMER (NCT02966834), a multicenter, double-blind, randomized, placebo-controlled trial, recruited 147 patients with PBC and moderate-to-severe pruritus. Following 4 weeks single-blind placebo, patients (randomized 3:1) received linerixibat or placebo for 12 weeks (to Week 16). Participants graded their itch (twice daily) and its interference with sleep (once daily) in an electronic diary using a 0-10 numerical rating scale (NRS). Weekly and monthly itch scores were calculated as the mean of the worst daily itch score over the respective time period. At study visits, participants completed the 5-D itch scale and the PBC-40 quality of life questionnaire, both of which contain an item specific to itch-related sleep disturbance. The impact of pruritus on sleep was assessed post hoc through correlations between the changes in NRS, 5-D itch, and PBC-40.
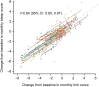
Results: Strong correlations were found between change from baseline in weekly itch and sleep NRS scores (r = 0.88 [95% confidence interval (CI): 0.83; 0.91]) at the end of treatment (Week 16), as well as in monthly itch and sleep NRS scores (r = 0.84 [95% CI: 0.80; 0.87]). Patients with improved weekly pruritus score severity category demonstrated reduced perceived sleep interference on average. Itch responders (≥2-point improvement in weekly itch score from baseline) displayed larger improvements in weekly sleep NRS score, 5-D itch, and PBC-40 sleep items, than itch non-responders (<2-point improvement).
Conclusions: A strong correlation exists between changes in pruritus severity and sleep interference in patients with PBC; pruritus reduction could generate concomitant improvement in sleep.
Keywords: Cholestatic pruritus; Health-related quality of life; IBAT inhibitor; PBC; Primary biliary cholangitis; Sleep interference.
© 2024. The Author(s).
Conflict of interest statement
RVM is an employee of GSK and holds GSK shares. MJM has received research support from CymaBay, GSK, Intercept, Mallinckrodt, Salix, and TARGET RWE; and has consulted for CymaBay, GSK, Mallinckrodt, Mirum, and TARGET RWE. HTS is an employee of GSK and holds GSK shares. AT was an employee of GSK at the time of the study and holds GSK shares. SD is an employee of GSK. ARdS is an employee of GSK and holds GSK shares. EL is an employee of GSK and holds GSK shares. CL is an ABIM committee member and an associate editor of
Figures



Similar articles
-
Psychometric validation of the Worst Itch Numerical Rating Scale (WI-NRS) and other patient-reported outcome measures for assessing severity and impact of pruritus in patients with primary biliary cholangitis.Orphanet J Rare Dis. 2025 Jul 31;20(1):390. doi: 10.1186/s13023-025-03798-x. Orphanet J Rare Dis. 2025. PMID: 40745549 Free PMC article.
-
Pruritus in Chronic Cholestatic Liver Diseases, Especially in Primary Biliary Cholangitis: A Narrative Review.Int J Mol Sci. 2025 Feb 22;26(5):1883. doi: 10.3390/ijms26051883. Int J Mol Sci. 2025. PMID: 40076514 Free PMC article. Review.
-
GLIMMER: A Randomized Phase 2b Dose-Ranging Trial of Linerixibat in Primary Biliary Cholangitis Patients With Pruritus.Clin Gastroenterol Hepatol. 2023 Jul;21(7):1902-1912.e13. doi: 10.1016/j.cgh.2022.10.032. Epub 2022 Nov 4. Clin Gastroenterol Hepatol. 2023. PMID: 36343847 Clinical Trial.
-
Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study.Lancet. 2017 Mar 18;389(10074):1114-1123. doi: 10.1016/S0140-6736(17)30319-7. Epub 2017 Feb 8. Lancet. 2017. PMID: 28187915 Clinical Trial.
-
New Treatment Paradigms in Primary Biliary Cholangitis.Clin Gastroenterol Hepatol. 2023 Jul;21(8):2076-2087. doi: 10.1016/j.cgh.2023.02.005. Epub 2023 Feb 19. Clin Gastroenterol Hepatol. 2023. PMID: 36809835 Review.
Cited by
-
Pervasive role of pruritus in impaired quality of life in patients with primary biliary cholangitis: Data from the GLIMMER study.Hepatol Commun. 2025 Feb 19;9(3):e0635. doi: 10.1097/HC9.0000000000000635. eCollection 2025 Mar 1. Hepatol Commun. 2025. PMID: 39969430 Free PMC article. Clinical Trial.
-
Elevated Bile Acids Induce Circadian Rhythm Sleep Disorders in Chronic Liver Diseases.Cell Mol Gastroenterol Hepatol. 2025;19(3):101439. doi: 10.1016/j.jcmgh.2024.101439. Epub 2024 Dec 10. Cell Mol Gastroenterol Hepatol. 2025. PMID: 39667579 Free PMC article.
-
Psychometric validation of the Worst Itch Numerical Rating Scale (WI-NRS) and other patient-reported outcome measures for assessing severity and impact of pruritus in patients with primary biliary cholangitis.Orphanet J Rare Dis. 2025 Jul 31;20(1):390. doi: 10.1186/s13023-025-03798-x. Orphanet J Rare Dis. 2025. PMID: 40745549 Free PMC article.
-
Pruritus in Chronic Cholestatic Liver Diseases, Especially in Primary Biliary Cholangitis: A Narrative Review.Int J Mol Sci. 2025 Feb 22;26(5):1883. doi: 10.3390/ijms26051883. Int J Mol Sci. 2025. PMID: 40076514 Free PMC article. Review.
References
-
- UK-PBC Consortium. Hegade VS, Mells GF, Fisher H, Kendrick S, DiBello J, Gilchrist K, Alexander GJ, Hirschfield GM, Sandford RN, Jones DEJ. Pruritus is common and undertreated in patients with primary biliary cholangitis in the United Kingdom. Clin Gastroenterol Hepatol. 2019;17(7):1379–1387. doi: 10.1016/j.cgh.2018.12.007. - DOI - PubMed
-
- (JSG-NAFLD) JSGoNFLD. Oeda S, Takahashi H, Yoshida H, Ogawa Y, Imajo K, Yoneda M, Koshiyama Y, Ono M, Hyogo H, Kawaguchi T, Fujii H, Nishino K, Sumida Y, Tanaka S, Kawanaka M, Torimura T, Saibara T, Kawaguchi A, Nakajima A, Eguchi Y. Prevalence of pruritus in patients with chronic liver disease: a multicenter study. Hepatol Res. 2018;48(3):E252–E262. doi: 10.1111/hepr.12978. - DOI - PubMed
-
- Mayo MJ, Carey E, Smith HT, Mospan AR, McLaughlin M, Thompson A, Morris HL, Sandefur R, Kim WR, Bowlus C, TARGET- PBC Investigators, Levy C. Impact of pruritus on quality of life and current treatment patterns in patients with primary biliary cholangitis. Dig Dis Sci. 2023;68(3):995–1005. doi: 10.1007/s10620-022-07581-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

