Deep-learning-enabled antibiotic discovery through molecular de-extinction
- PMID: 38862735
- PMCID: PMC11310081
- DOI: 10.1038/s41551-024-01201-x
Deep-learning-enabled antibiotic discovery through molecular de-extinction
Abstract
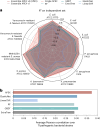
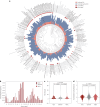
Molecular de-extinction aims at resurrecting molecules to solve antibiotic resistance and other present-day biological and biomedical problems. Here we show that deep learning can be used to mine the proteomes of all available extinct organisms for the discovery of antibiotic peptides. We trained ensembles of deep-learning models consisting of a peptide-sequence encoder coupled with neural networks for the prediction of antimicrobial activity and used it to mine 10,311,899 peptides. The models predicted 37,176 sequences with broad-spectrum antimicrobial activity, 11,035 of which were not found in extant organisms. We synthesized 69 peptides and experimentally confirmed their activity against bacterial pathogens. Most peptides killed bacteria by depolarizing their cytoplasmic membrane, contrary to known antimicrobial peptides, which tend to target the outer membrane. Notably, lead compounds (including mammuthusin-2 from the woolly mammoth, elephasin-2 from the straight-tusked elephant, hydrodamin-1 from the ancient sea cow, mylodonin-2 from the giant sloth and megalocerin-1 from the extinct giant elk) showed anti-infective activity in mice with skin abscess or thigh infections. Molecular de-extinction aided by deep learning may accelerate the discovery of therapeutic molecules.
© 2024. The Author(s).
Conflict of interest statement
C.F.-N. provides consulting services to Invaio Sciences and is a member of the Scientific Advisory Boards of Nowture S.L., Peptidus and Phare Bio. The de la Fuente Lab has received research funding or in-kind donations from United Therapeutics, Strata Manufacturing PJSC and Procter & Gamble, none of which were used in support of this work.
Figures




References
-
- New Report Calls for Urgent Actionto Avert Antimicrobial Resistance Crisis (World Health Organization, 2019).
-
- Zuckerkandl, E. & Pauling, L. Molecules as documents of evolutionary history. J. Theor. Biol.8, 357–366 (1965). - PubMed
MeSH terms
Substances
Grants and funding
- R35 GM138201/GM/NIGMS NIH HHS/United States
- HDTRA11810041/United States Department of Defense | Defense Threat Reduction Agency (DTRA)
- R35GM138201/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- HDTRA1-23-1-0001/United States Department of Defense | Defense Threat Reduction Agency (DTRA)
- HDTRA1-21-1-0014/United States Department of Defense | Defense Threat Reduction Agency (DTRA)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

