Impact of gallbladder hypoplasia on hilar hepatic ducts in biliary atresia
- PMID: 38862768
- PMCID: PMC11166647
- DOI: 10.1038/s43856-024-00544-5
Impact of gallbladder hypoplasia on hilar hepatic ducts in biliary atresia
Abstract
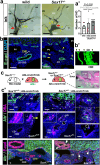
Background: Biliary atresia (BA) is an intractable disease of unknown cause that develops in the neonatal period. It causes jaundice and liver damage due to the destruction of extrahepatic biliary tracts,. We have found that heterozygous knockout mice of the SRY related HMG-box 17 (Sox17) gene, a master regulator of stem/progenitor cells in the gallbladder wall, exhibit a condition like BA. However, the precise contribution of hypoplastic gallbladder wall to the pathogenesis of hepatobiliary disease in Sox17 heterozygous embryos and human BA remains unclear.
Methods: We employed cholangiography and histological analyses in the mouse BA model. Furthermore, we conducted a retrospective analysis of human BA.
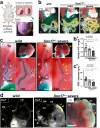
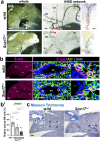
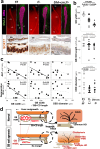
Results: We show that gallbladder wall hypoplasia causes abnormal multiple connections between the hilar hepatic bile ducts and the gallbladder-cystic duct in Sox17 heterozygous embryos. These multiple hilar extrahepatic ducts fuse with the developing intrahepatic duct walls and pull them out of the liver parenchyma, resulting in abnormal intrahepatic duct network and severe cholestasis. In human BA with gallbladder wall hypoplasia (i.e., abnormally reduced expression of SOX17), we also identify a strong association between reduced gallbladder width (a morphometric parameter indicating gallbladder wall hypoplasia) and severe liver injury at the time of the Kasai surgery, like the Sox17-mutant mouse model.
Conclusions: Together with the close correlation between gallbladder wall hypoplasia and liver damage in both mouse and human cases, these findings provide an insight into the critical role of SOX17-positive gallbladder walls in establishing functional bile duct networks in the hepatic hilus of neonates.
Plain language summary
Biliary atresia (BA) is a disease in newborns that causes a serious liver condition due to damage to the bile ducts (the pathways that carry bile juice). Although reduced function of a key gene called Sox17, which is essential for forming the gallbladder wall, has been observed in some BA cases, the link between gallbladder issues and liver damage is unknown. This study has shown how damage spreads through the bile ducts in the liver around the time of birth when there are problems in the gallbladder wall due to reduced SOX17 function. The findings indicate that proper growth of the gallbladder wall during this critical period is essential for forming a normal network of bile ducts in the developing liver. This discovery is promising for early diagnosis and better treatment of BA in newborns.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Gallbladder wall abnormality in biliary atresia of mouse Sox17+/- neonates and human infants.Dis Model Mech. 2020 Apr 3;13(4):dmm042119. doi: 10.1242/dmm.042119. Dis Model Mech. 2020. PMID: 31996362 Free PMC article.
-
Anatomical and histological characteristics of the hepatobiliary system in adult Sox17 heterozygote mice.Anat Rec (Hoboken). 2020 Dec;303(12):3096-3107. doi: 10.1002/ar.24466. Epub 2020 Jul 2. Anat Rec (Hoboken). 2020. PMID: 32478476
-
A case series of prenatal hepatic hilar cyst in the presence of a gallbladder - navigating the dilemma between biliary atresia and choledochal cyst.BMC Pediatr. 2024 Sep 13;24(1):580. doi: 10.1186/s12887-024-05043-z. BMC Pediatr. 2024. PMID: 39272011 Free PMC article.
-
Practical approach to imaging diagnosis of biliary atresia, Part 1: prenatal ultrasound and magnetic resonance imaging, and postnatal ultrasound.Pediatr Radiol. 2021 Feb;51(2):314-331. doi: 10.1007/s00247-020-04840-9. Epub 2020 Nov 17. Pediatr Radiol. 2021. PMID: 33201318
-
Biliary parasitic diseases including clonorchiasis, opisthorchiasis and fascioliasis.Abdom Imaging. 2008 Mar-Apr;33(2):157-65. doi: 10.1007/s00261-007-9326-x. Abdom Imaging. 2008. PMID: 17934771 Review.
Cited by
-
Immunoregulation role of the erythroid cells.Front Immunol. 2024 Oct 15;15:1466669. doi: 10.3389/fimmu.2024.1466669. eCollection 2024. Front Immunol. 2024. PMID: 39474425 Free PMC article. Review.
-
Versatile application of fast green FCF as a visible cholangiogram in adult mice to medium-sized mammals.Sci Rep. 2025 Jan 16;15(1):1960. doi: 10.1038/s41598-024-84355-7. Sci Rep. 2025. PMID: 39821095 Free PMC article.
-
Genetic background and biliary atresia.World J Pediatr Surg. 2025 Jun 6;8(3):e001023. doi: 10.1136/wjps-2025-001023. eCollection 2025. World J Pediatr Surg. 2025. PMID: 40519535 Free PMC article. Review.
References
Grants and funding
- 24228005/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20H00445/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22J11160/MEXT | Japan Society for the Promotion of Science (JSPS)
- 18K14583/MEXT | Japan Society for the Promotion of Science (JSPS)
- JPMJSP2108/MEXT | Japan Science and Technology Agency (JST)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

