Effects of Kv1.3 knockout on pyramidal neuron excitability and synaptic plasticity in piriform cortex of mice
- PMID: 38862816
- PMCID: PMC11420205
- DOI: 10.1038/s41401-024-01275-y
Effects of Kv1.3 knockout on pyramidal neuron excitability and synaptic plasticity in piriform cortex of mice
Abstract
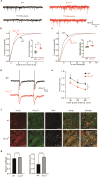
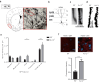
Kv1.3 belongs to the voltage-gated potassium (Kv) channel family, which is widely expressed in the central nervous system and associated with a variety of neuropsychiatric disorders. Kv1.3 is highly expressed in the olfactory bulb and piriform cortex and involved in the process of odor perception and nutrient metabolism in animals. Previous studies have explored the function of Kv1.3 in olfactory bulb, while the role of Kv1.3 in piriform cortex was less known. In this study, we investigated the neuronal changes of piriform cortex and feeding behavior after smell stimulation, thus revealing a link between the olfactory sensation and body weight in Kv1.3 KO mice. Coronal slices including the anterior piriform cortex were prepared, whole-cell recording and Ca2+ imaging of pyramidal neurons were conducted. We showed that the firing frequency evoked by depolarization pulses and Ca2+ influx evoked by high K+ solution were significantly increased in pyramidal neurons of Kv1.3 knockout (KO) mice compared to WT mice. Western blotting and immunofluorescence analyses revealed that the downstream signaling molecules CaMKII and PKCα were activated in piriform cortex of Kv1.3 KO mice. Pyramidal neurons in Kv1.3 KO mice exhibited significantly reduced paired-pulse ratio and increased presynaptic Cav2.1 expression, proving that the presynaptic vesicle release might be elevated by Ca2+ influx. Using Golgi staining, we found significantly increased dendritic spine density of pyramidal neurons in Kv1.3 KO mice, supporting the stronger postsynaptic responses in these neurons. In olfactory recognition and feeding behavior tests, we showed that Kv1.3 conditional knockout or cannula injection of 5-(4-phenoxybutoxy) psoralen, a Kv1.3 channel blocker, in piriform cortex both elevated the olfactory recognition index and altered the feeding behavior in mice. In summary, Kv1.3 is a key molecule in regulating neuronal activity of the piriform cortex, which may lay a foundation for the treatment of diseases related to piriform cortex and olfactory detection.
Keywords: Kv1.3; neuronal excitability; olfactory recognition; piriform cortex; synaptic plasticity.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur J Neurosci. 1995;7:2189–205. - PubMed
-
- Zhao W, Chen Y. Progress in research of KV1.1 and KV1.3 channels as therapeutic targets. Curr Top Med Chem. 2016;16:1877–85. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

