Overexpression of PavHIPP16 from Prunus avium enhances cold stress tolerance in transgenic tobacco
- PMID: 38862890
- PMCID: PMC11167810
- DOI: 10.1186/s12870-024-05267-2
Overexpression of PavHIPP16 from Prunus avium enhances cold stress tolerance in transgenic tobacco
Abstract
Background: The heavy metal-associated isoprenylated plant protein (HIPP) is an important regulatory element in response to abiotic stresses, especially playing a key role in low-temperature response.
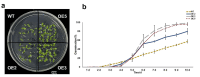
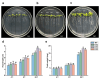
Results: This study investigated the potential function of PavHIPP16 up-regulated in sweet cherry under cold stress by heterologous overexpression in tobacco. The results showed that the overexpression (OE) lines' growth state was better than wild type (WT), and the germination rate, root length, and fresh weight of OE lines were significantly higher than those of WT. In addition, the relative conductivity and malondialdehyde (MDA) content of the OE of tobacco under low-temperature treatment were substantially lower than those of WT. In contrast, peroxidase (POD), superoxide dismutase (SOD), catalase (CAT) activities, hydrogen peroxide (H2O2), proline, soluble protein, and soluble sugar contents were significantly higher than those of WT. Yeast two-hybrid assay (Y2H) and luciferase complementation assay verified the interactions between PavbHLH106 and PavHIPP16, suggesting that these two proteins co-regulated the cold tolerance mechanism in plants. The research results indicated that the transgenic lines could perform better under low-temperature stress by increasing the antioxidant enzyme activity and osmoregulatory substance content of the transgenic plants.
Conclusions: This study provides genetic resources for analyzing the biological functions of PavHIPPs, which is important for elucidating the mechanisms of cold resistance in sweet cherry.
Keywords: Genetic transformation; HIPP; Low-temperature stress; Protein interaction; Sweet cherry.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Molecular identification and expression patterns of sweet cherry HIPPs and functional analysis of PavHIPP16 in cold stress.Planta. 2024 Nov 6;260(6):134. doi: 10.1007/s00425-024-04567-z. Planta. 2024. PMID: 39505755
-
Functional analysis of sweet cherry PavbHLH106 in the regulation of cold stress.Plant Cell Rep. 2023 Dec 22;43(1):7. doi: 10.1007/s00299-023-03115-5. Plant Cell Rep. 2023. PMID: 38133822
-
Overexpression of PavbHLH28 from Prunus avium enhances tolerance to cold stress in transgenic Arabidopsis.BMC Plant Biol. 2023 Dec 18;23(1):652. doi: 10.1186/s12870-023-04666-1. BMC Plant Biol. 2023. PMID: 38110865 Free PMC article.
-
Genome-wide identification of cold shock proteins (CSPs) in sweet cherry (Prunus avium L.) and exploring the differential responses of PavCSP1 and PavCSP3 to low temperature and salt stress.Genes Genomics. 2024 Sep;46(9):1023-1036. doi: 10.1007/s13258-024-01542-6. Epub 2024 Jul 12. Genes Genomics. 2024. PMID: 38997611
-
HcLEA113, a late embryogenesis abundant protein gene, positively regulates drought-stress responses in kenaf.Physiol Plant. 2024 Jul-Aug;176(4):e14506. doi: 10.1111/ppl.14506. Physiol Plant. 2024. PMID: 39191701
Cited by
-
McWRKY43 Confers Cold Stress Tolerance in Michelia crassipes via Regulation of Flavonoid Biosynthesis.Int J Mol Sci. 2024 Sep 12;25(18):9843. doi: 10.3390/ijms25189843. Int J Mol Sci. 2024. PMID: 39337331 Free PMC article.
References
-
- Barth O, Vogt S, Uhlemann R, Zschiesche W, Humbeck K. Stress induced and nuclear localized HIPP26 from Arabidopsis thaliana interacts via its heavy metal associated domain with the drought stress related zinc finger transcription factor ATHB29. Plant Mol Biol. 2009;69:213–26. doi: 10.1007/s11103-008-9419-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- Grant No. 32160700/the National Natural Science Foundation of China
- Grant No. YQK[2023]008/he Guizhou Provincial Science and Technology Projects of China
- Grant No. [2023]009/the National Guidance of Local Science and Technology Development Fund of China
- Grant No. [2021] Yiban231/the Guizhou Provincial Science and Technology Projects of China
LinkOut - more resources
Full Text Sources
Miscellaneous

