Effects of a multicomponent communication training to involve older people in decisions to DEPRESCRIBE cardiometabolic medication in primary care (CO-DEPRESCRIBE): protocol for a cluster randomized controlled trial with embedded process and economic evaluation
- PMID: 38862899
- PMCID: PMC11165805
- DOI: 10.1186/s12875-024-02465-7
Effects of a multicomponent communication training to involve older people in decisions to DEPRESCRIBE cardiometabolic medication in primary care (CO-DEPRESCRIBE): protocol for a cluster randomized controlled trial with embedded process and economic evaluation
Abstract
Background: Deprescribing of medication for cardiovascular risk factors and diabetes has been incorporated in clinical guidelines but proves to be difficult to implement in primary care. Training of healthcare providers is needed to enhance deprescribing in eligible patients. This study will examine the effects of a blended training program aimed at initiating and conducting constructive deprescribing consultations with patients.
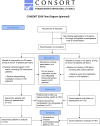
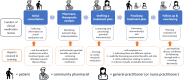
Methods: A cluster-randomized trial will be conducted in which local pharmacy-general practice teams in the Netherlands will be randomized to conducting clinical medication reviews with patients as usual (control) or after receiving the CO-DEPRESCRIBE training program (intervention). People of 75 years and older using specific cardiometabolic medication (diabetes drugs, antihypertensives, statins) and eligible for a medication review will be included. The CO-DEPRESCRIBE intervention is based on previous work and applies models for patient-centered communication and shared decision making. It consists of 5 training modules with supportive tools. The primary outcome is the percentage of patients with at least 1 cardiometabolic medication deintensified. Secondary outcomes include patient involvement in decision making, healthcare provider communication skills, health/medication-related outcomes, attitudes towards deprescribing, medication regimen complexity and health-related quality of life. Additional safety and cost parameters will be collected. It is estimated that 167 patients per study arm are needed in the final intention-to-treat analysis using a mixed effects model. Taking loss to follow-up into account, 40 teams are asked to recruit 10 patients each. A baseline and 6-months follow-up assessment, a process evaluation, and a cost-effectiveness analysis will be conducted.
Discussion: The hypothesis is that the training program will lead to more proactive and patient-centered deprescribing of cardiometabolic medication. By a comprehensive evaluation, an increase in knowledge needed for sustainable implementation of deprescribing in primary care is expected.
Trial registration: The study is registered at ClinicalTrials.gov (identifier: NCT05507177).
Keywords: Barriers- facilitators; Deprescribing; Patient-centered care; Primary care; Provider-patient communication; Shared decision making.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Perspectives on deprescribing in older people with type 2 diabetes and/or cardiovascular conditions: challenges from healthcare provider, patient and caregiver perspective, and interventions to support a proactive approach.Expert Rev Clin Pharmacol. 2024 Aug;17(8):637-654. doi: 10.1080/17512433.2024.2378765. Epub 2024 Aug 12. Expert Rev Clin Pharmacol. 2024. PMID: 39119644 Review.
-
Barriers and Enablers of Healthcare Providers to Deprescribe Cardiometabolic Medication in Older Patients: A Focus Group Study.Drugs Aging. 2022 Mar;39(3):209-221. doi: 10.1007/s40266-021-00918-7. Epub 2022 Feb 21. Drugs Aging. 2022. PMID: 35187614 Free PMC article.
-
Effectiveness of a multi-faceted intervention to deprescribe proton pump inhibitors in primary care: protocol for a population-based, pragmatic, cluster-randomized controlled trial.BMC Health Serv Res. 2022 Feb 17;22(1):219. doi: 10.1186/s12913-022-07496-3. BMC Health Serv Res. 2022. PMID: 35177042 Free PMC article.
-
Deprescribing as a Way to Reduce Inappropriate Use of Drugs for Overactive Bladder in Primary Care (DROP): Protocol for a Cluster Randomized Controlled Trial With an Embedded Explanatory Sequential Mixed Methods Study.JMIR Res Protoc. 2024 Jul 23;13:e56277. doi: 10.2196/56277. JMIR Res Protoc. 2024. PMID: 39042875 Free PMC article.
-
What makes a multidisciplinary medication review and deprescribing intervention for older people work well in primary care? A realist review and synthesis.BMC Geriatr. 2023 Sep 25;23(1):591. doi: 10.1186/s12877-023-04256-8. BMC Geriatr. 2023. PMID: 37743469 Free PMC article. Review.
References
-
- Nederlands Huisartsen Genootschap i.s.m. andere beroepsorganisaties/instanties/ verenigingen [Dutch College of General Practitioners in collaboration with other professional organisations].Module Minderen En Stoppen, Onderdeel van de Multidisciplinaire Richtlijn Polyfarmacie Bij Ouderen [Module Deprescribing, part of the Multidisciplinary Guideline Polypharmacy in Elderly]. 2020; (December). https://richtlijnen.nhg.org//files/2020-11/Final_Module%20Minderen%20en%....
-
- Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–34. - PubMed
-
- Christiaens A, Henrard S, Sinclair AJ, Tubach F, Bonnet-Zamponi D, Zerah L. Deprescribing glucose-lowering therapy in older adults with diabetes: a systematic review of recommendations. J Am Med Dir Assoc. 2023;24(3):400–2. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical