Xinbao Pill ameliorates heart failure via regulating the SGLT1/AMPK/PPARα axis to improve myocardial fatty acid energy metabolism
- PMID: 38862959
- PMCID: PMC11165817
- DOI: 10.1186/s13020-024-00959-1
Xinbao Pill ameliorates heart failure via regulating the SGLT1/AMPK/PPARα axis to improve myocardial fatty acid energy metabolism
Abstract
Background: Heart failure (HF) is characterized by a disorder of cardiomyocyte energy metabolism. Xinbao Pill (XBW), a traditional Chinese medicine formulation integrating "Liushen Pill" and "Shenfu Decoction," has been approved by China Food and Drug Administration for the treatment of HF for many years. The present study reveals a novel mechanism of XBW in HF through modulation of cardiac energy metabolism.
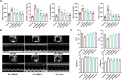
Methods: In vivo, XBW (60, 90, 120 mg/kg/d) and fenofibrate (100 mg/kg/d) were treated for six weeks in Sprague-Dawley rats that were stimulated by isoproterenol to induce HF. Cardiac function parameters were measured by echocardiography, and cardiac pathological changes were assessed using H&E, Masson, and WGA staining. In vitro, primary cultured neonatal rat cardiomyocytes (NRCMs) were induced by isoproterenol to investigate the effects of XBW on myocardial cell damage, mitochondrial function and fatty acid energy metabolism. The involvement of the SGLT1/AMPK/PPARα signalling axis was investigated.
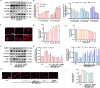
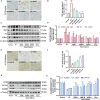
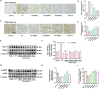
Results: In both in vitro and in vivo models of ISO-induced HF, XBW significantly ameliorated cardiac hypertrophy cardiac fibrosis, and improved cardiac function. Significantly, XBW improved cardiac fatty acid metabolism and mitigated mitochondrial damage. Mechanistically, XBW effectively suppressed the expression of SGLT1 protein while upregulating the phosphorylation level of AMPK, ultimately facilitating the nuclear translocation of PPARα and enhancing its transcriptional activity. Knockdown of SGLT1 further enhanced cardiac energy metabolism by XBW, while overexpression of SGLT1 reversed the cardio-protective effect of XBW, highlighting that SGLT1 is probably a critical target of XBW in the regulation of cardiac fatty acid metabolism.
Conclusions: XBW improves cardiac fatty acid energy metabolism to alleviate HF via SGLT1/AMPK/PPARα signalling axis.
Keywords: AMPK/PPARα axis; Heart failure; Myocardial energy metabolism; SGLT1; Xinbao Pill.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Cinnamaldehyde activates AMPK/PGC-1α pathway via targeting GRK2 to ameliorate heart failure.Phytomedicine. 2024 Oct;133:155894. doi: 10.1016/j.phymed.2024.155894. Epub 2024 Jul 20. Phytomedicine. 2024. PMID: 39089090
-
Integrated chemical profiling, network pharmacology and pharmacological evaluation to explore the potential mechanism of Xinbao pill against myocardial ischaemia-reperfusion injury.Pharm Biol. 2022 Dec;60(1):255-273. doi: 10.1080/13880209.2022.2025859. Pharm Biol. 2022. PMID: 35148221 Free PMC article.
-
Ginsenoside Rb1 promotes the activation of PPARα pathway via inhibiting FADD to ameliorate heart failure.Eur J Pharmacol. 2023 May 15;947:175676. doi: 10.1016/j.ejphar.2023.175676. Epub 2023 Mar 30. Eur J Pharmacol. 2023. PMID: 37001580
-
Ginsenoside Rb1 Ameliorates Heart Failure Ventricular Remodeling by Regulating the Twist1/PGC-1α/PPARα Signaling Pathway.Pharmaceuticals (Basel). 2025 Mar 30;18(4):500. doi: 10.3390/ph18040500. Pharmaceuticals (Basel). 2025. PMID: 40283937 Free PMC article.
-
Modulation of fatty acid metabolism is involved in the alleviation of isoproterenol-induced rat heart failure by fenofibrate.Mol Med Rep. 2015 Dec;12(6):7899-906. doi: 10.3892/mmr.2015.4466. Epub 2015 Oct 21. Mol Med Rep. 2015. PMID: 26497978 Free PMC article.
Cited by
-
Magnesium-assisted hydrogen improves isoproterenol-induced heart failure.Med Gas Res. 2025 Dec 1;15(4):459-470. doi: 10.4103/mgr.MEDGASRES-D-24-00135. Epub 2025 Apr 29. Med Gas Res. 2025. PMID: 40300881 Free PMC article.
-
Sex-Specific Antioxidant and Anti-Inflammatory Protective Effects of AMPK in Cardiovascular Diseases.Antioxidants (Basel). 2025 May 21;14(5):615. doi: 10.3390/antiox14050615. Antioxidants (Basel). 2025. PMID: 40427496 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

