De novo design of a nanoregulator for the dynamic restoration of ovarian tissue in cryopreservation and transplantation
- PMID: 38862987
- PMCID: PMC11167790
- DOI: 10.1186/s12951-024-02602-5
De novo design of a nanoregulator for the dynamic restoration of ovarian tissue in cryopreservation and transplantation
Abstract
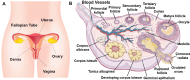
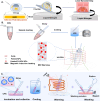
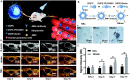
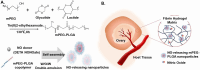
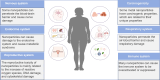
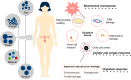
The cryopreservation and transplantation of ovarian tissue underscore its paramount importance in safeguarding reproductive capacity and ameliorating reproductive disorders. However, challenges persist in ovarian tissue cryopreservation and transplantation (OTC-T), including the risk of tissue damage and dysfunction. Consequently, there has been a compelling exploration into the realm of nanoregulators to refine and enhance these procedures. This review embarks on a meticulous examination of the intricate anatomical structure of the ovary and its microenvironment, thereby establishing a robust groundwork for the development of nanomodulators. It systematically categorizes nanoregulators and delves deeply into their functions and mechanisms, meticulously tailored for optimizing ovarian tissue cryopreservation and transplantation. Furthermore, the review imparts valuable insights into the practical applications and obstacles encountered in clinical settings associated with OTC-T. Moreover, the review advocates for the utilization of microbially derived nanomodulators as a potent therapeutic intervention in ovarian tissue cryopreservation. The progression of these approaches holds the promise of seamlessly integrating nanoregulators into OTC-T practices, thereby heralding a new era of expansive applications and auspicious prospects in this pivotal domain.
Keywords: Nanoparticle; Nanoregulators; Ovarian system; Ovarian tissue cryopreservation and transplantation; Reproductive system; Transplantation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Del Castillo LM, Buigues A, Rossi V, Soriano MJ, Martinez J, De Felici M, et al. The cyto-protective effects of LH on ovarian reserve and female fertility during exposure to gonadotoxic alkylating agents in an adult mouse model. Hum Reprod. 2021;36:2514–2528. doi: 10.1093/humrep/deab165. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- BX202208/Sichuan Province Innovative Talent Funding Project for Postdoctoral Fellows
- 2023ZYD0083/Central Government Guides Local Science and Technology Development Fund Projects
- 2023ZYD0116/Central Government Guides Local Science and Technology Development Fund Projects
- 21ZD009/Key Medical Science and Technology Research and Development Project of Sichuan Provincial Health Commission
- 2023YFS0057/Sichuan Provincial Science and Technology Plan Project
LinkOut - more resources
Full Text Sources

