Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy
- PMID: 38863020
- PMCID: PMC11167803
- DOI: 10.1186/s13045-024-01559-0
Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy
Abstract
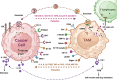
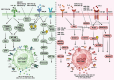
Macrophages infiltrating tumour tissues or residing in the microenvironment of solid tumours are known as tumour-associated macrophages (TAMs). These specialized immune cells play crucial roles in tumour growth, angiogenesis, immune regulation, metastasis, and chemoresistance. TAMs encompass various subpopulations, primarily classified into M1 and M2 subtypes based on their differentiation and activities. M1 macrophages, characterized by a pro-inflammatory phenotype, exert anti-tumoural effects, while M2 macrophages, with an anti-inflammatory phenotype, function as protumoural regulators. These highly versatile cells respond to stimuli from tumour cells and other constituents within the tumour microenvironment (TME), such as growth factors, cytokines, chemokines, and enzymes. These stimuli induce their polarization towards one phenotype or another, leading to complex interactions with TME components and influencing both pro-tumour and anti-tumour processes.This review comprehensively and deeply covers the literature on macrophages, their origin and function as well as the intricate interplay between macrophages and the TME, influencing the dual nature of TAMs in promoting both pro- and anti-tumour processes. Moreover, the review delves into the primary pathways implicated in macrophage polarization, examining the diverse stimuli that regulate this process. These stimuli play a crucial role in shaping the phenotype and functions of macrophages. In addition, the advantages and limitations of current macrophage based clinical interventions are reviewed, including enhancing TAM phagocytosis, inducing TAM exhaustion, inhibiting TAM recruitment, and polarizing TAMs towards an M1-like phenotype. In conclusion, while the treatment strategies targeting macrophages in precision medicine show promise, overcoming several obstacles is still necessary to achieve an accessible and efficient immunotherapy.
Keywords: Cancer cell; Immunity; Immunotherapy; Polarization; Tumour microenvironment; Tumour-associated macrophages.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Mechanistic studies of tumor-associated macrophage immunotherapy.Front Immunol. 2024 Sep 30;15:1476565. doi: 10.3389/fimmu.2024.1476565. eCollection 2024. Front Immunol. 2024. PMID: 39403370 Free PMC article. Review.
-
Tumor-associated macrophages: A sentinel of innate immune system in tumor microenvironment gone haywire.Cell Biol Int. 2024 Oct;48(10):1406-1449. doi: 10.1002/cbin.12226. Epub 2024 Jul 25. Cell Biol Int. 2024. PMID: 39054741 Review.
-
Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy.Front Immunol. 2022 Jun 30;13:888713. doi: 10.3389/fimmu.2022.888713. eCollection 2022. Front Immunol. 2022. PMID: 35844605 Free PMC article. Review.
-
Tumor-associated macrophages remodel the suppressive tumor immune microenvironment and targeted therapy for immunotherapy.J Exp Clin Cancer Res. 2025 May 16;44(1):145. doi: 10.1186/s13046-025-03377-9. J Exp Clin Cancer Res. 2025. PMID: 40380196 Free PMC article. Review.
-
Macrophage-based cancer immunotherapy: Challenges and opportunities.Exp Cell Res. 2024 Sep 1;442(1):114198. doi: 10.1016/j.yexcr.2024.114198. Epub 2024 Aug 3. Exp Cell Res. 2024. PMID: 39103071 Review.
Cited by
-
Advanced Nanoprobe Strategies for Imaging Macrophage Polarization in Cancer Immunology.Research (Wash D C). 2025 Feb 21;8:0622. doi: 10.34133/research.0622. eCollection 2025. Research (Wash D C). 2025. PMID: 39990770 Free PMC article. Review.
-
Exploring the roles and clinical potential of exosome-derived non-coding RNAs in glioma.IBRO Neurosci Rep. 2025 Feb 5;18:323-337. doi: 10.1016/j.ibneur.2025.01.015. eCollection 2025 Jun. IBRO Neurosci Rep. 2025. PMID: 40034544 Free PMC article. Review.
-
Sphingosine kinase 1 promotes M2 macrophage infiltration and enhances glioma cell migration via the JAK2/STAT3 pathway.Sci Rep. 2025 Feb 4;15(1):4152. doi: 10.1038/s41598-025-88328-2. Sci Rep. 2025. PMID: 39900970 Free PMC article.
-
Optimizing CAR-T cell therapy for solid tumors: current challenges and potential strategies.J Hematol Oncol. 2024 Nov 5;17(1):105. doi: 10.1186/s13045-024-01625-7. J Hematol Oncol. 2024. PMID: 39501358 Free PMC article. Review.
-
Decoding meningioma prognosis with multi-omics: macrophage diversity, immune-CNV interplay, and novel SPP1-targeted strategies.J Neurooncol. 2025 Jun 16. doi: 10.1007/s11060-025-05116-8. Online ahead of print. J Neurooncol. 2025. PMID: 40522564
References
-
- Kvansakul M, Hinds MG. The molecular basis of chemotherapy resistance in cancer. Oncogene. 2021;40(45):6199–215.
Publication types
MeSH terms
Grants and funding
- CA2116 E-COST-GRANT/Short-Term Scientific Mission
- 10383, 2023/Award of EMBO Scientific Exchange Grant
- P18-FR-2470/Consejería de Economía, Conocimiento, Empresas y Universidad de la Junta de Andalucía and European Regional Development Fund (ERDF)
- RTI 2018-101309-B-C22/Ministerio de Ciencia, Innovación y Universidades
- CMC-CTS963/Chair "Doctors Galera-Requena in cancer stem cell research"
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

