Validated chromatographic approach for determination of two ternary mixtures in newly approved formulations for helicobacter pylori eradication: assessment of greenness profile and content uniformity
- PMID: 38863068
- PMCID: PMC11167897
- DOI: 10.1186/s13065-024-01215-1
Validated chromatographic approach for determination of two ternary mixtures in newly approved formulations for helicobacter pylori eradication: assessment of greenness profile and content uniformity
Abstract
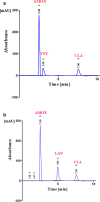
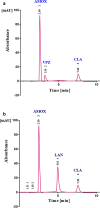
A new, sensitive, and rapid isocratic reversed phase chromatographic method (RP-HPLC-UV) was developed for simultaneous separation of two newly co-formulated antiulcer mixtures; Amoxicillin, Vonoprazan and Clarithromycin [Mixture (I)], and Amoxicillin, Lansoprazole and Clarithromycin [Mixture (II)]. Analytical separation was performed using a Promosil C18 column and ultraviolet detection at 210 nm. The separation was achieved within only 8 min. For both mixtures, an aqueous solution, composed of (Acetonitrile: Methanol: 0. 2 M phosphoric acid) within ratio of (30: 30: 40) adjusted to final pH 3.0, was the mobile phase. This method was validated as per the International Conference on Harmonization guidelines. The linearity ranges of these proposed method of the (Mixture (I)) were 25.0-400.0 µg/mL Amoxicillin, 0.5-8.0 µg/mL Vonoprazan, and 12.5-200.0 µg/mL Clarithromycin. And the linearity ranges of the (Mixture (II)) were 10.0-300.0 µg/mL Amoxicillin, 0.3-9.0 µg/mL Lansoprazole and 5.0-150.0 µg/mL Clarithromycin. This method was firstly applied for effective separation of Amoxicillin, Vonoprazan and Clarithromycin [Mixture (I)]. It fulfilled good repeatability, sensitivity, and accuracy (R.S.D. < 2.0%). The mean recoveries of the analytes in their Tri-Pak formulations were acceptable. The greenness of the developed chromatographic methods was assessed using an Eco-scale method and it was applied for content uniformity testing as per the United States Pharmacopoeia (USP) and the acceptance value of Amoxicillin, in Mixture (I) was 2.88, the acceptance values for Amoxicillin, Lansoprazole in Mixture (II) were 2.592, 2.424, respectively.
Keywords: Amoxicillin; Capsule; Clarithromycin; Content uniformity; Eco-scale; Lansoprazole; Liquid chromatographic method; Tri-Pak; Vonoprazan.
© 2024. The Author(s).
Conflict of interest statement
The authors have no conflict of interest. The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence this work.
Figures
Similar articles
-
RP-HPLC-DAD Method Development and Validation for Simultaneous Determination of Lansoprazole, Tinidazole, Amoxicillin, and Naproxen in Their Raw Materials and Combined Dosage Form: DOE Approach for Optimization of the Proposed Method.J AOAC Int. 2022 Apr 27;105(3):675-687. doi: 10.1093/jaoacint/qsab159. J AOAC Int. 2022. PMID: 34918094
-
An eco-friendly liquid chromatographic analysis of the triple therapy protocol of amoxicillin, metronidazole and vonoprazan for H. Pylori eradication: application to combined dosage forms and simulated gastric fluid.BMC Chem. 2024 May 30;18(1):106. doi: 10.1186/s13065-024-01210-6. BMC Chem. 2024. PMID: 38816886 Free PMC article.
-
An eco-friendly multi-analyte high-performance thin layer chromatographic densitometric determination of amoxicillin, metronidazole, and famotidine in their ternary mixtures and simulated gastric juice: A promising protocol for eradicating Helicobacter pylori.J Sep Sci. 2023 Feb;46(4):e2200951. doi: 10.1002/jssc.202200951. Epub 2022 Dec 22. J Sep Sci. 2023. PMID: 36524974
-
Efficacy and Safety of Vonoprazan and Amoxicillin Dual Therapy for Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis.Digestion. 2023;104(4):249-261. doi: 10.1159/000529622. Epub 2023 Apr 4. Digestion. 2023. PMID: 37015201 Free PMC article.
-
Vonoprazan With Amoxicillin or Amoxicillin and Clarithromycin for the Treatment of Helicobacter pylori Infection.Ann Pharmacother. 2023 Oct;57(10):1185-1197. doi: 10.1177/10600280221149708. Epub 2023 Jan 23. Ann Pharmacother. 2023. PMID: 36688309 Review.
References
LinkOut - more resources
Full Text Sources
