Colchicine to reduce coronavirus disease-19-related inflammation and cardiovascular complications in high-risk patients post-acute infection with SARS-COV-2-a study protocol for a randomized controlled trial
- PMID: 38863076
- PMCID: PMC11167886
- DOI: 10.1186/s13063-024-08205-7
Colchicine to reduce coronavirus disease-19-related inflammation and cardiovascular complications in high-risk patients post-acute infection with SARS-COV-2-a study protocol for a randomized controlled trial
Abstract
Background: There is no known effective pharmacological therapy for long COVID, which is characterized by wide-ranging, multisystemic, fluctuating, or relapsing symptoms in a large proportion of survivors of acute COVID. This randomized controlled trial aims to assess the safety and efficacy of an anti-inflammatory agent colchicine, to reduce symptoms among those at high risk of developing long COVID.
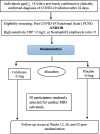
Methods: This multi-centre, parallel arm, 1:1 individual randomized, placebo-controlled, double-blind superiority trial will enrol 350 individuals with persistent post-COVID symptoms. Participants will be randomized to either colchicine 0.5 mg once daily (< 70 kg) or twice daily (≥ 70 kg) or matched placebo for 26 weeks and will be followed up until 52 weeks after randomization. The primary trial objective is to demonstrate the superiority of colchicine over a placebo in improving distance walked in 6 min at 52 weeks from baseline. The secondary objectives are to assess the efficacy of colchicine compared to placebo with respect to lung function, inflammatory markers, constitutional symptoms, and mental health state. In a sub-sample of 100 participants, cardiac biomarkers of myocardial injury and myocardial oedema using MRI will be compared.
Discussion: Persistent inflammatory response following SARS-CoV-19 is one of the postulated pathophysiological mechanisms of long COVID. Colchicine, a low-cost anti-inflammatory agent, acts via multiple inflammatory pathways and has an established safety profile. This trial will generate evidence for an important health priority that can rapidly translate into practice.
Trial registration: This clinical trial has been registered prospectively on www.
Clinicaltrials: gov with registration CTRI/2021/11/038234 dated November 24, 2021.
Keywords: Colchicine; Inflammation; Long COVID; Lung function; Randomized controlled trial.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Similar articles
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
The SARS-CoV-2 Ivermectin Navarra-ISGlobal Trial (SAINT) to Evaluate the Potential of Ivermectin to Reduce COVID-19 Transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: A structured summary of a study protocol for a randomized control pilot trial.Trials. 2020 Jun 8;21(1):498. doi: 10.1186/s13063-020-04421-z. Trials. 2020. PMID: 32513289 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Anti-inflammatory therapy for COVID-19 infection: the case for colchicine.Ann Rheum Dis. 2021 May;80(5):550-557. doi: 10.1136/annrheumdis-2020-219174. Epub 2020 Dec 8. Ann Rheum Dis. 2021. PMID: 33293273 Free PMC article. Review.
-
Colchicine: A potential therapeutic tool against COVID-19. Experience of 5 patients.Reumatol Clin (Engl Ed). 2021 Aug-Sep;17(7):371-375. doi: 10.1016/j.reumae.2020.05.008. Reumatol Clin (Engl Ed). 2021. PMID: 34301378 Free PMC article. Review.
References
-
- WHO. Weekly epidemiological update on COVID-19 2023 https://www.who.int/publications/m/item/weekly-epidemiological-update-on.... Accessed 22 Jun 2023
-
- WHO. A clinical case definition of post COVID-19 condition by a Delphi consensus. World Health organization 2021 https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_cond.... Accessed 6 Oct 2021
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous