Enlarged perivascular spaces in the basal ganglia are associated with arteries not veins
- PMID: 38863151
- PMCID: PMC11542128
- DOI: 10.1177/0271678X241260629
Enlarged perivascular spaces in the basal ganglia are associated with arteries not veins
Abstract
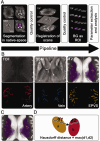
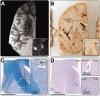
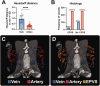
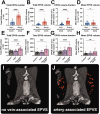
Enlarged perivascular spaces (EPVS) are common in cerebral small vessel disease (CSVD) and have been identified as a marker of dysfunctional brain clearance. However, it remains unknown if the enlargement occurs predominantly around arteries or veins. We combined in vivo ultra-high-resolution MRI and histopathology to investigate the spatial relationship of veins and arteries with EPVS within the basal ganglia (BG). Furthermore, we assessed the relationship between the EPVS and measures of blood-flow (blood-flow velocity, pulsatility index) in the small arteries of the BG. Twenty-four healthy controls, twelve non-CAA CSVD patients, and five probable CAA patients underwent a 3 tesla [T] and 7T MRI-scan, and EPVS, arteries, and veins within the BG were manually segmented. Furthermore, the scans were co-registered. Six autopsy-cases were also assessed. In the BG, EPVS were significantly closer to and overlapped more frequently with arteries than with veins. Histological analysis showed a higher proportion of BG EPVS surrounding arteries than veins. Finally, the pulsatility index of BG arteries correlated with EPVS volume. Our results are in line with previous works and establish a pathophysiological relationship between arteries and EPVS, contributing to elucidating perivascular clearance routes in the human brain.
Keywords: 7T MRI; cerebral small vessel disease; histopathology; hypertensive arteriopathy; perivascular clearance.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Impact of enlarged perivascular spaces in the basal ganglia on gait in cerebral small vessel disease.Aging Clin Exp Res. 2025 Apr 30;37(1):138. doi: 10.1007/s40520-025-03045-0. Aging Clin Exp Res. 2025. PMID: 40304928 Free PMC article.
-
The relationship between blood-brain barrier permeability and enlarged perivascular spaces: a cross-sectional study.Clin Interv Aging. 2019 May 10;14:871-878. doi: 10.2147/CIA.S204269. eCollection 2019. Clin Interv Aging. 2019. PMID: 31190773 Free PMC article.
-
Enlarged Perivascular Spaces Are Independently Associated with High Pulse Wave Velocity: A Cross-Sectional Study.J Alzheimers Dis. 2024;101(2):627-636. doi: 10.3233/JAD-240589. J Alzheimers Dis. 2024. PMID: 39213072 Free PMC article.
-
Factors associated with the location of perivascular space enlargement in middle-aged individuals undergoing brain screening in Japan.Clin Neurol Neurosurg. 2022 Dec;223:107497. doi: 10.1016/j.clineuro.2022.107497. Epub 2022 Nov 2. Clin Neurol Neurosurg. 2022. PMID: 36356441 Review.
-
Enlarged Perivascular Spaces and Dementia: A Systematic Review.J Alzheimers Dis. 2019;72(1):247-256. doi: 10.3233/JAD-190527. J Alzheimers Dis. 2019. PMID: 31561362 Free PMC article.
Cited by
-
Visualization of perivascular spaces in the human brain with 5-T magnetic resonance imaging.BMC Neurosci. 2025 Mar 3;26(1):18. doi: 10.1186/s12868-025-00925-z. BMC Neurosci. 2025. PMID: 40033208 Free PMC article.
-
Free water as a potential mediator linking basal ganglia peri-vascular spaces to white matter hyperintensities in cerebral small vessel disease.Front Neurosci. 2025 Jul 3;19:1621023. doi: 10.3389/fnins.2025.1621023. eCollection 2025. Front Neurosci. 2025. PMID: 40678759 Free PMC article.
-
Robust, fully-automated assessment of cerebral perivascular spaces and white matter lesions: a multicentre MRI longitudinal study of their evolution and association with risk of dementia and accelerated brain atrophy.EBioMedicine. 2025 Jan;111:105523. doi: 10.1016/j.ebiom.2024.105523. Epub 2024 Dec 24. EBioMedicine. 2025. PMID: 39721217 Free PMC article.
References
-
- Virchow R. Über die erweiterung kleinerer gefäße. Virchows Arch 1851; 3: 427–462.
-
- Robin C. Recherches sur quelques particularites de la structure des capillaires de l’encephale. J Physiol Homme Anim 1859; 2: 537–548.
-
- Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020; 16: 137–153. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

