Oxygen fluctuations in the brain and periphery induced by intravenous fentanyl: effects of dose and drug experience
- PMID: 38863429
- PMCID: PMC11427041
- DOI: 10.1152/jn.00177.2024
Oxygen fluctuations in the brain and periphery induced by intravenous fentanyl: effects of dose and drug experience
Abstract
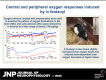
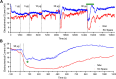
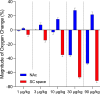
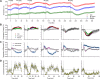
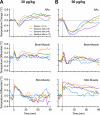
Fentanyl is the leading contributor to drug overdose deaths in the United States. Its potency, rapid onset of action, and lack of effective reversal treatment make the drug much more lethal than other opioids. Although it is understood that fentanyl is dangerous at higher doses, the literature surrounding fentanyl's physiological effects remains contradictory at lower doses. To explore this discrepancy, we designed a study incorporating electrochemical assessment of oxygen in the brain (nucleus accumbens) and subcutaneous space, multisite thermorecording (brain, skin, muscle), and locomotor activity at varying doses of fentanyl (1.0, 3.0, 10, 30, and 90 µg/kg) in rats. In the nucleus accumbens, lower doses of fentanyl (3.0 and 10 µg/kg) led to an increase in oxygen levels while higher doses (30 and 90 µg/kg) led to a biphasic pattern, with an initial dose-dependent decrease followed by an increase. In the subcutaneous space, oxygen decreases started to appear at relatively lower doses (>3 µg/kg), had shorter onset latencies, and were stronger and prolonged. In the temperature experiment, lower doses of fentanyl (1.0, 3.0, and 10 µg/kg) led to an increase in brain, skin, and muscle temperatures, while higher doses (30 and 90 µg/kg) resulted in a dose-dependent biphasic temperature change, with an increase followed by a prolonged decrease. We also compared oxygen and temperature responses induced by fentanyl over six consecutive days and found no evidence of tolerance in both parameters. In conclusion, we report that fentanyl's effects are highly dose-dependent, drawing attention to the importance of better characterization to adequately respond in emergent cases of illicit fentanyl misuse.NEW & NOTEWORTHY By using electrochemical oxygen sensors in freely moving rats, we show that intravenous fentanyl induces opposite changes in brain oxygen at varying doses, increasing at lower doses (<10 µg/kg) and inducing a biphasic response, decrease followed by increase, at higher doses (>10-90 µg/kg). In contrast, fentanyl-induced dose-dependent oxygen decreases in the subcutaneous space. We consider the mechanisms underlying distinct oxygen responses in the brain and periphery and discuss naloxone's role in alleviating fentanyl-induced brain hypoxia.
Keywords: brain hyperoxia; central vasodilation; hyperthermia; hypothermia; peripheral vasoconstriction.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Comment in
-
The complexity of fentanyl's impact on brain and peripheral oxygenation.J Neurophysiol. 2024 Aug 1;132(2):319-321. doi: 10.1152/jn.00256.2024. Epub 2024 Jun 19. J Neurophysiol. 2024. PMID: 38896832 No abstract available.
References
-
- Jaffe JH, Knapp CM, Ciraulo DA. Opiates: clinical aspects. In: Substance Abuse (3rd ed.), edited by Lowinson JH, Ruiz P, Millman RB, Langrod JG. Baltimore, MD: Williams & Wilkins, 2005, p. 158–166.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

