Staphylococcal mastitis in dairy cows
- PMID: 38863450
- PMCID: PMC11165426
- DOI: 10.3389/fvets.2024.1356259
Staphylococcal mastitis in dairy cows
Abstract
Bovine mastitis is one of the most common diseases of dairy cattle. Even though different infectious microorganisms and mechanical injury can cause mastitis, bacteria are the most common cause of mastitis in dairy cows. Staphylococci, streptococci, and coliforms are the most frequently diagnosed etiological agents of mastitis in dairy cows. Staphylococci that cause mastitis are broadly divided into Staphylococcus aureus and non-aureus staphylococci (NAS). NAS is mainly comprised of coagulase-negative Staphylococcus species (CNS) and some coagulase-positive and coagulase-variable staphylococci. Current staphylococcal mastitis control measures are ineffective, and dependence on antimicrobial drugs is not sustainable because of the low cure rate with antimicrobial treatment and the development of resistance. Non-antimicrobial effective and sustainable control tools are critically needed. This review describes the current status of S. aureus and NAS mastitis in dairy cows and flags areas of knowledge gaps.
Keywords: Staphylococcus aureus; antimicrobial resistance of staphylococci; bovine staphylococcal mastitis; control; dairy cow; host immune responses; non-aureus staphylococci; staphylococcal virulence factors.
Copyright © 2024 Kerro Dego and Vidlund.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
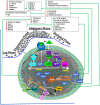
Similar articles
-
Distinct behavior of bovine-associated staphylococci species in their ability to resist phagocytosis and trigger respiratory burst activity by blood and milk polymorphonuclear leukocytes in dairy cows.J Dairy Sci. 2022 Feb;105(2):1625-1637. doi: 10.3168/jds.2021-20953. Epub 2021 Nov 19. J Dairy Sci. 2022. PMID: 34802732
-
Molecular epidemiology and distribution of antimicrobial resistance genes of Staphylococcus species isolated from Chinese dairy cows with clinical mastitis.J Dairy Sci. 2019 Feb;102(2):1571-1583. doi: 10.3168/jds.2018-15136. Epub 2018 Dec 24. J Dairy Sci. 2019. PMID: 30591326
-
Distribution of non-aureus staphylococci from quarter milk, teat apices, and rectal feces of dairy cows, and their virulence potential.J Dairy Sci. 2020 Nov;103(11):10658-10675. doi: 10.3168/jds.2020-18265. Epub 2020 Sep 10. J Dairy Sci. 2020. PMID: 32921446
-
Coagulase-negative staphylococci as cause of bovine mastitis- not so different from Staphylococcus aureus?Vet Microbiol. 2009 Feb 16;134(1-2):29-36. doi: 10.1016/j.vetmic.2008.09.011. Epub 2008 Sep 11. Vet Microbiol. 2009. PMID: 18977615 Review.
-
Coagulase-negative staphylococci-emerging mastitis pathogens.Vet Microbiol. 2009 Feb 16;134(1-2):3-8. doi: 10.1016/j.vetmic.2008.09.015. Epub 2008 Sep 11. Vet Microbiol. 2009. PMID: 18848410 Review.
Cited by
-
Antimicrobial Susceptibility Patterns of Staphylococcus spp. Isolates from Mastitic Cases in Romanian Buffaloes from Western Romania.Antibiotics (Basel). 2025 May 23;14(6):537. doi: 10.3390/antibiotics14060537. Antibiotics (Basel). 2025. PMID: 40558127 Free PMC article.
-
Phage Endolysins as an Alternative Biocontrol Strategy for Pathogenic and Spoilage Microorganisms in the Food Industry.Viruses. 2025 Apr 14;17(4):564. doi: 10.3390/v17040564. Viruses. 2025. PMID: 40285007 Free PMC article. Review.
-
Annual and Seasonal Trends in Mastitis Pathogens Isolated from Milk Samples from Dairy Cows of California's San Joaquin Valley Dairies Between January 2009 and December 2023.Vet Sci. 2025 Jun 21;12(7):609. doi: 10.3390/vetsci12070609. Vet Sci. 2025. PMID: 40711269 Free PMC article.
-
Clonality, Virulence Genes, and Antimicrobial Resistance of Dairy Ruminants in Mastitic Milk-Associated Staphylococcus aureus in Sicily.Antibiotics (Basel). 2025 Feb 12;14(2):188. doi: 10.3390/antibiotics14020188. Antibiotics (Basel). 2025. PMID: 40001431 Free PMC article.
-
Characteristic profiles of molecular types, antibiotic resistance, antibiotic resistance genes, and virulence genes of Staphylococcus aureus isolates from caprine mastitis in China.Front Cell Infect Microbiol. 2025 Feb 18;15:1533844. doi: 10.3389/fcimb.2025.1533844. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40041149 Free PMC article.
References
-
- Bramley AJ, National Mastitis C. NMC. (1996). Current concepts of bovine mastitis, 4th ed. Madison, WI: National Mastitis Council.
-
- USDA APHIS Veterinary Services (VS), the Center for Epidemiology and Animal Health (CEAH). Prevalence of Contagious Mastitis Pathogens on US dairy operations, 2007, Info sheet, Fort Collins, CO (2008). Available at: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy07/D... (Accessed April 26, 2024).
Publication types
LinkOut - more resources
Full Text Sources

