Smart responsive in situ hydrogel systems applied in bone tissue engineering
- PMID: 38863497
- PMCID: PMC11165218
- DOI: 10.3389/fbioe.2024.1389733
Smart responsive in situ hydrogel systems applied in bone tissue engineering
Abstract
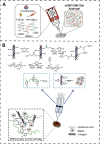
The repair of irregular bone tissue suffers severe clinical problems due to the scarcity of an appropriate therapeutic carrier that can match dynamic and complex bone damage. Fortunately, stimuli-responsive in situ hydrogel systems that are triggered by a special microenvironment could be an ideal method of regenerating bone tissue because of the injectability, in situ gelatin, and spatiotemporally tunable drug release. Herein, we introduce the two main stimulus-response approaches, exogenous and endogenous, to forming in situ hydrogels in bone tissue engineering. First, we summarize specific and distinct responses to an extensive range of external stimuli (e.g., ultraviolet, near-infrared, ultrasound, etc.) to form in situ hydrogels created from biocompatible materials modified by various functional groups or hybrid functional nanoparticles. Furthermore, "smart" hydrogels, which respond to endogenous physiological or environmental stimuli (e.g., temperature, pH, enzyme, etc.), can achieve in situ gelation by one injection in vivo without additional intervention. Moreover, the mild chemistry response-mediated in situ hydrogel systems also offer fascinating prospects in bone tissue engineering, such as a Diels-Alder, Michael addition, thiol-Michael addition, and Schiff reactions, etc. The recent developments and challenges of various smart in situ hydrogels and their application to drug administration and bone tissue engineering are discussed in this review. It is anticipated that advanced strategies and innovative ideas of in situ hydrogels will be exploited in the clinical field and increase the quality of life for patients with bone damage.
Keywords: bone tissue engineering; endogenous stimulus; exogenous stimulus; in situ hydrogels; smart hydrogels.
Copyright © 2024 Wu, Gai, Chen, Chen and Chen.
Conflict of interest statement
Author SW was employed by Hangzhou Singclean Medical Products Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures












Similar articles
-
Smart hydrogel-based trends in future tendon injury repair: A review.Int J Biol Macromol. 2024 Dec;282(Pt 5):137092. doi: 10.1016/j.ijbiomac.2024.137092. Epub 2024 Nov 1. Int J Biol Macromol. 2024. PMID: 39489238 Review.
-
Smart Hydrogels - Synthetic Stimuli-Responsive Antitumor Drug Release Systems.Int J Nanomedicine. 2020 Jun 25;15:4541-4572. doi: 10.2147/IJN.S248987. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32617004 Free PMC article. Review.
-
Advances of Stimulus-Responsive Hydrogels for Bone Defects Repair in Tissue Engineering.Gels. 2022 Jun 20;8(6):389. doi: 10.3390/gels8060389. Gels. 2022. PMID: 35735733 Free PMC article. Review.
-
Decoupled pH- and Thermo-Responsive Injectable Chitosan/PNIPAM Hydrogel via Thiol-Ene Click Chemistry for Potential Applications in Tissue Engineering.Adv Healthc Mater. 2020 Jul;9(14):e2000454. doi: 10.1002/adhm.202000454. Epub 2020 Jun 16. Adv Healthc Mater. 2020. PMID: 32548983
-
Three-Dimensional Printing and Injectable Conductive Hydrogels for Tissue Engineering Application.Tissue Eng Part B Rev. 2019 Oct;25(5):398-411. doi: 10.1089/ten.TEB.2019.0100. Epub 2019 Sep 11. Tissue Eng Part B Rev. 2019. PMID: 31115274 Review.
Cited by
-
Engineered Hydrogels for Musculoskeletal Regeneration: Advanced Synthesis Strategies and Therapeutic Efficacy in Preclinical Models.Polymers (Basel). 2025 Jul 30;17(15):2094. doi: 10.3390/polym17152094. Polymers (Basel). 2025. PMID: 40808142 Free PMC article. Review.
-
A review of synergistic strategies in cancer therapy: resveratrol-loaded hydrogels for targeted and multimodal treatment.Discov Oncol. 2025 Jul 21;16(1):1382. doi: 10.1007/s12672-025-03079-w. Discov Oncol. 2025. PMID: 40690143 Free PMC article. Review.
-
Environmental stimuli-responsive hydrogels in endodontics: Advances and perspectives.Int Endod J. 2025 May;58(5):674-684. doi: 10.1111/iej.14208. Epub 2025 Feb 6. Int Endod J. 2025. PMID: 39915932 Free PMC article. Review.
-
Bone Regeneration: Mini-Review and Appealing Perspectives.Bioengineering (Basel). 2025 Jan 7;12(1):38. doi: 10.3390/bioengineering12010038. Bioengineering (Basel). 2025. PMID: 39851312 Free PMC article. Review.
References
-
- Abueva C. D. G., Chung P.-S., Ryu H.-S., Park S.-Y., Woo S. H. (2020). Photoresponsive hydrogels as drug delivery systems. Med. Lasers 9 (1), 6–11. 10.25289/ML.2020.9.1.6 - DOI
Publication types
LinkOut - more resources
Full Text Sources

