Unveiling the protective role of anthocyanin in rice: insights into drought-induced oxidative stress and metabolic regulation
- PMID: 38863532
- PMCID: PMC11165195
- DOI: 10.3389/fpls.2024.1397817
Unveiling the protective role of anthocyanin in rice: insights into drought-induced oxidative stress and metabolic regulation
Abstract
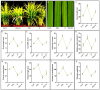
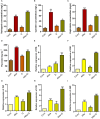
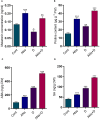
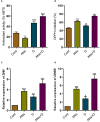
This study investigates the impact of anthocyanin treatment on rice plants under drought stress, focusing on phenotypic, molecular, and biochemical responses. Anthocyanin were treated to one month old plants one week before the droughtexposure. Drought stress was imposed by using 10% polyethylene glycol (PEG 6000). Anthocyanin-treated plants exhibited significant enhancements in various traits, including growth parameters and reproductive characteristics, under normal conditions. When subjected to drought stress, these plants displayed resilience, maintaining or improving essential morphological and physiological features compared to non-treated counterparts. Notably, anthocyanin application mitigated drought-induced oxidative stress, as evidenced by reduced levels of reactive oxygen species (ROS) and lipid membrane peroxidation. The study also elucidates the regulatory role of anthocyanins in the expression of flavonoid biosynthetic genes, leading to increased levels of key secondary metabolites. Furthermore, anthocyanin treatment influenced the levels of stress-related signaling molecules, including melatonin, proline, abscisic acid (ABA), and salicylic acid (SA), contributing to enhanced stress tolerance. The enzymatic activity of antioxidants and the expression of drought-responsive genes were modulated by anthocyanins, emphasizing their role in antioxidant defense and stress response. Additionally, anthocyanin treatment positively influenced macronutrient concentrations, particularly calcium ion (Ca+), potassium ion (K+), and sodium ion (Na+), essential for cell wall and membrane stability. The findings collectively highlight the multifaceted protective effects of anthocyanins, positioning them as potential key players in conferring resilience to drought stress in rice plants. The study provides valuable insights into the molecular and physiological mechanisms underlying anthocyanin-mediated enhancement of drought stress tolerance, suggesting promising applications in agricultural practices for sustainable crop production.
Keywords: abscisic acid; anthocyanin; antioxidants; drought stress; melatonin.
Copyright © 2024 Jan, Asif, Asaf, Lubna, Khan and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Age-related mechanism and its relationship with secondary metabolism and abscisic acid in Aristotelia chilensis plants subjected to drought stress.Plant Physiol Biochem. 2018 Mar;124:136-145. doi: 10.1016/j.plaphy.2018.01.010. Epub 2018 Jan 12. Plant Physiol Biochem. 2018. PMID: 29360623
-
Evaluating the involvement and interaction of abscisic acid and miRNA156 in the induction of anthocyanin biosynthesis in drought-stressed plants.Planta. 2017 Aug;246(2):299-312. doi: 10.1007/s00425-017-2711-y. Epub 2017 May 22. Planta. 2017. PMID: 28534253 Review.
-
Nitric oxide and abscisic acid protects against PEG-induced drought stress differentially in Brassica genotypes by combining the role of stress modulators, markers and antioxidants.Nitric Oxide. 2019 Aug 1;89:81-92. doi: 10.1016/j.niox.2019.05.005. Epub 2019 May 13. Nitric Oxide. 2019. PMID: 31096008
-
Overexpression of BnMYBL2-1 improves plant drought tolerance via the ABA-dependent pathway.Plant Physiol Biochem. 2024 Feb;207:108293. doi: 10.1016/j.plaphy.2023.108293. Epub 2023 Dec 22. Plant Physiol Biochem. 2024. PMID: 38181638
-
The Role of Anthocyanins in Plant Tolerance to Drought and Salt Stresses.Plants (Basel). 2023 Jul 5;12(13):2558. doi: 10.3390/plants12132558. Plants (Basel). 2023. PMID: 37447119 Free PMC article. Review.
Cited by
-
Occurrence, Biosynthesis, and Health Benefits of Anthocyanins in Rice and Barley.Int J Mol Sci. 2025 Jun 27;26(13):6225. doi: 10.3390/ijms26136225. Int J Mol Sci. 2025. PMID: 40649998 Free PMC article. Review.
-
Comparative transcriptome and metabolome profiling unveil genotype-specific strategies for drought tolerance in cotton.Front Plant Sci. 2025 Jun 13;16:1610552. doi: 10.3389/fpls.2025.1610552. eCollection 2025. Front Plant Sci. 2025. PMID: 40584853 Free PMC article.
-
Bioactive compounds in extracts from short rotation willow shoots known as pharmaceuticals and experimental demonstration of biostimulation of maize plants by these chemical complexes.Front Plant Sci. 2025 Aug 4;16:1650824. doi: 10.3389/fpls.2025.1650824. eCollection 2025. Front Plant Sci. 2025. PMID: 40831725 Free PMC article.
-
Unveiling physiological responses and modulated accumulation patterns of specialized metabolites in Mentha rotundifolia acclimated to sub-tropical environment.Physiol Mol Biol Plants. 2024 Aug;30(8):1363-1381. doi: 10.1007/s12298-024-01489-8. Epub 2024 Jul 19. Physiol Mol Biol Plants. 2024. PMID: 39184553 Free PMC article.
-
Antioxidant Activity of Anthocyanins and Anthocyanidins: A Critical Review.Int J Mol Sci. 2024 Nov 8;25(22):12001. doi: 10.3390/ijms252212001. Int J Mol Sci. 2024. PMID: 39596068 Free PMC article. Review.
References
-
- Adhikari B., Dhungana S. K., Ali M. W., Adhikari A., Kim I.-D., Shin D.-H. (2019). Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. J. Saudi Soc. Agric. Sci. 18, 437–442. doi: 10.1016/j.jssas.2018.02.004 - DOI
-
- Bashir S. S., Hussain A., Hussain S. J., Wani O. A., Zahid Nabi S., Dar N. A., et al. . (2021). Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 35, 1912–1925. doi: 10.1080/13102818.2021.2020161 - DOI
-
- Brito C., Dinis L.-T., Meijón M., Ferreira H., Pinto G., Moutinho-Pereira J., et al. . (2018). Salicylic acid modulates olive tree physiological and growth responses to drought and rewatering events in a dose dependent manner. J. Plant Physiol. 230, 21–32. doi: 10.1016/j.jplph.2018.08.004 - DOI - PubMed
LinkOut - more resources
Full Text Sources

