Single-molecule characterization of salivary protein aggregates from Parkinson's disease patients: a pilot study
- PMID: 38863577
- PMCID: PMC11166177
- DOI: 10.1093/braincomms/fcae178
Single-molecule characterization of salivary protein aggregates from Parkinson's disease patients: a pilot study
Abstract
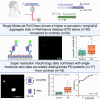
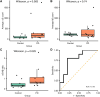
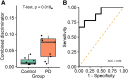
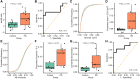
Saliva is a convenient and accessible biofluid that has potential as a future diagnostic tool for Parkinson's disease. Candidate diagnostic tests for Parkinson's disease to date have predominantly focused on measurements of α-synuclein in CSF, but there is a need for accurate tests utilizing more easily accessible sample types. Prior studies utilizing saliva have used bulk measurements of salivary α-synuclein to provide diagnostic insight. Aggregate structure may influence the contribution of α-synuclein to disease pathology. Single-molecule approaches can characterize the structure of individual aggregates present in the biofluid and may, therefore, provide greater insight than bulk measurements. We have employed an antibody-based single-molecule pulldown assay to quantify salivary α-synuclein and amyloid-β peptide aggregate numbers and subsequently super-resolved captured aggregates using direct Stochastic Optical Reconstruction Microscopy to describe their morphological features. We show that the salivary α-synuclein aggregate/amyloid-β aggregate ratio is increased almost 2-fold in patients with Parkinson's disease (n = 20) compared with controls (n = 20, P < 0.05). Morphological information also provides insight, with saliva from patients with Parkinson's disease containing a greater proportion of larger and more fibrillar amyloid-β aggregates than control saliva (P < 0.05). Furthermore, the combination of count and morphology data provides greater diagnostic value than either measure alone, distinguishing between patients with Parkinson's disease (n = 17) and controls (n = 18) with a high degree of accuracy (area under the curve = 0.87, P < 0.001) and a larger dynamic range. We, therefore, demonstrate for the first time the application of highly sensitive single-molecule imaging techniques to saliva. In addition, we show that aggregates present within saliva retain relevant structural information, further expanding the potential utility of saliva-based diagnostic methods.
Keywords: Parkinson’s disease; amyloid-β; saliva; single-molecule imaging; α-synuclein.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures



Similar articles
-
Imaging protein aggregates in the serum and cerebrospinal fluid in Parkinson's disease.Brain. 2022 Apr 18;145(2):632-643. doi: 10.1093/brain/awab306. Brain. 2022. PMID: 34410317 Free PMC article.
-
A systematic review of salivary biomarkers in Parkinson's disease.Neural Regen Res. 2024 Dec 1;19(12):2613-2625. doi: 10.4103/NRR.NRR-D-23-01677. Epub 2024 Mar 1. Neural Regen Res. 2024. PMID: 38595280 Free PMC article.
-
Single-Molecule Imaging of Individual Amyloid Protein Aggregates in Human Biofluids.ACS Chem Neurosci. 2016 Mar 16;7(3):399-406. doi: 10.1021/acschemneuro.5b00324. Epub 2016 Feb 4. ACS Chem Neurosci. 2016. PMID: 26800462 Free PMC article.
-
In search of Parkinson's disease biomarkers - is the answer in our mouths? A systematic review of the literature on salivary biomarkers of Parkinson's disease.Neurol Neurochir Pol. 2020;54(1):14-20. doi: 10.5603/PJNNS.a2020.0011. Epub 2020 Jan 31. Neurol Neurochir Pol. 2020. PMID: 32003440
-
Insights into the Molecular Mechanisms of Alzheimer's and Parkinson's Diseases with Molecular Simulations: Understanding the Roles of Artificial and Pathological Missense Mutations in Intrinsically Disordered Proteins Related to Pathology.Int J Mol Sci. 2018 Jan 24;19(2):336. doi: 10.3390/ijms19020336. Int J Mol Sci. 2018. PMID: 29364151 Free PMC article. Review.
Cited by
-
Improved Imaging Surface for Quantitative Single-Molecule Microscopy.ACS Appl Mater Interfaces. 2024 Jul 17;16(28):37255-37264. doi: 10.1021/acsami.4c06512. Epub 2024 Jul 9. ACS Appl Mater Interfaces. 2024. PMID: 38979642 Free PMC article.
-
ASC specks as a single-molecule fluid biomarker of inflammation in neurodegenerative diseases.Nat Commun. 2024 Nov 11;15(1):9690. doi: 10.1038/s41467-024-53547-0. Nat Commun. 2024. PMID: 39528447 Free PMC article.
References
-
- Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm. 2017;124(8):901–905. - PubMed
-
- Postuma RB, Berg D, Adler CH, et al. The new definition and diagnostic criteria of Parkinson’s disease. Lancet Neurol. 2016;15(6):546–548. - PubMed
-
- Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
