Resveratrol-Ampicillin Dual-Drug Loaded Polyvinylpyrrolidone/Polyvinyl Alcohol Biomimic Electrospun Nanofiber Enriched with Collagen for Efficient Burn Wound Repair
- PMID: 38863647
- PMCID: PMC11164821
- DOI: 10.2147/IJN.S464046
Resveratrol-Ampicillin Dual-Drug Loaded Polyvinylpyrrolidone/Polyvinyl Alcohol Biomimic Electrospun Nanofiber Enriched with Collagen for Efficient Burn Wound Repair
Abstract
Background: The healing of burn wounds is a complicated physiological process that involves several stages, including haemostasis, inflammation, proliferation, and remodelling to rebuild the skin and subcutaneous tissue integrity. Recent advancements in nanomaterials, especially nanofibers, have opened a new way for efficient healing of wounds due to burning or other injuries.
Methods: This study aims to develop and characterize collagen-decorated, bilayered electrospun nanofibrous mats composed of PVP and PVA loaded with Resveratrol (RSV) and Ampicillin (AMP) to accelerate burn wound healing and tissue repair.
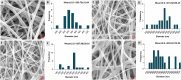
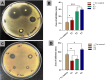
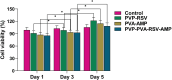
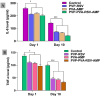
Results: Nanofibers with smooth surfaces and web-like structures with diameters ranging from 200 to 400 nm were successfully produced by electrospinning. These fibres exhibited excellent in vitro properties, including the ability to absorb wound exudates and undergo biodegradation over a two-week period. Additionally, these nanofibers demonstrated sustained and controlled release of encapsulated Resveratrol (RSV) and Ampicillin (AMP) through in vitro release studies. The zone of inhibition (ZOI) of PVP-PVA-RSV-AMP nanofibers against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) was found 31±0.09 mm and 12±0.03, respectively, which was significantly higher as compared to positive control. Similarly, the biofilm study confirmed the significant reduction in the formation of biofilms in nanofiber-treated group against both S. aureus and E. coli. X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) analysis proved the encapsulation of RSV and AMP successfully into nanofibers and their compatibility. Haemolysis assay (%) showed no significant haemolysis (less than 5%) in nanofiber-treated groups, confirmed their cytocompatibility with red blood cells (RBCs). Cell viability assay and cell adhesion on HaCaT cells showed increased cell proliferation, indicating its biocompatibility as well as non-toxic properties. Results of the in-vivo experiments on a burn wound model demonstrated potential burn wound healing in rats confirmed by H&E-stained images and also improved the collagen synthesis in nanofibers-treated groups evidenced by Masson-trichrome staining. The ELISA assay clearly indicated the efficient downregulation of TNF-alpha and IL-6 inflammatory biomarkers after treatment with nanofibers on day 10.
Conclusion: The RSV and AMP-loaded nanofiber mats, developed in this study, expedite burn wound healing through their multifaceted approach.
Keywords: ampicillin; burn wound healing; collagen; electrospinning; nanofiber; resveratrol.
© 2024 Kanaujiya et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures










Similar articles
-
Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing.Int J Biol Macromol. 2018 Dec;120(Pt A):385-393. doi: 10.1016/j.ijbiomac.2018.08.057. Epub 2018 Aug 12. Int J Biol Macromol. 2018. PMID: 30110603
-
Poly(vinyl alcohol)/Polycaprolactone Nanofiber Enriched with Lichenysin against Multidrug-Resistance Bacterial Infection in Wound Healing: In Vitro Studies and In Vivo Evaluation in Wistar Rats.ACS Appl Bio Mater. 2025 Apr 21;8(4):2847-2866. doi: 10.1021/acsabm.4c01532. Epub 2025 Mar 12. ACS Appl Bio Mater. 2025. PMID: 40074674
-
Sodium alginate-based nanofibers loaded with Capparis Sepiaria plant extract for wound healing.J Biomater Sci Polym Ed. 2024 Oct;35(15):2380-2401. doi: 10.1080/09205063.2024.2381375. Epub 2024 Jul 22. J Biomater Sci Polym Ed. 2024. PMID: 39037962
-
Nanodiamond: a multifaceted exploration of electrospun nanofibers for antibacterial and wound healing applications.J Nanobiotechnology. 2025 Apr 9;23(1):285. doi: 10.1186/s12951-025-03351-9. J Nanobiotechnology. 2025. PMID: 40205555 Free PMC article. Review.
-
Recent progress of electrospun nanofibers as burning dressings.RSC Adv. 2024 May 1;14(20):14374-14391. doi: 10.1039/d4ra01514b. eCollection 2024 Apr 25. RSC Adv. 2024. PMID: 38694552 Free PMC article. Review.
Cited by
-
Electrospun Nanofibers from Plant Natural Products: A New Approach Toward Efficient Wound Healing.Int J Nanomedicine. 2024 Dec 27;19:13973-13990. doi: 10.2147/IJN.S501970. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39742091 Free PMC article. Review.
-
HER-2 Receptor and αvβ3 Integrin Dual-Ligand Surface-Functionalized Liposome for Metastatic Breast Cancer Therapy.Pharmaceutics. 2024 Aug 27;16(9):1128. doi: 10.3390/pharmaceutics16091128. Pharmaceutics. 2024. PMID: 39339166 Free PMC article.
-
Therapeutic effect of pH responsive Magainin II modified azithromycin plus curcumin micelles in different depth models of MRSA infection.Sci Rep. 2025 Mar 3;15(1):7383. doi: 10.1038/s41598-025-92384-z. Sci Rep. 2025. PMID: 40025264 Free PMC article.
-
Accelerated Wound Healing of Tetrahedral-Framework Nucleic Acid Nanozymes with High Penetration and Antioxidant Capacity.Nanomaterials (Basel). 2024 Oct 23;14(21):1693. doi: 10.3390/nano14211693. Nanomaterials (Basel). 2024. PMID: 39513773 Free PMC article.
References
-
- Heo DN. Burn-wound healing effect of gelatin/polyurethane nanofiber scaffold containing silver-sulfadiazine. J Biomed Nanotechnol. 2013;9(3):511–515. - PubMed
-
- Yerra A, Dadala MMJJOAPS. Silk fibroin electrospun nanofiber blends with antibiotics and polyvinyl alcohol for burn wound healing. J Appl Poly Sci. 2022;139(15):51930.
-
- Talukder ME. Novel fibrin functionalized multilayered electrospun nanofiber membrane for burn wound treatment. J Mat Sci. 2021;56(22):12814–12834.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

