Immunization with PfGBP130 generates antibodies that inhibit RBC invasion by P. falciparum parasites
- PMID: 38863702
- PMCID: PMC11165087
- DOI: 10.3389/fimmu.2024.1350560
Immunization with PfGBP130 generates antibodies that inhibit RBC invasion by P. falciparum parasites
Abstract
Background: Despite decades of effort, Plasmodium falciparum malaria remains a leading killer of children. The absence of a highly effective vaccine and the emergence of parasites resistant to both diagnosis as well as treatment hamper effective public health interventions.
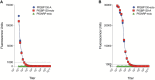
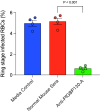
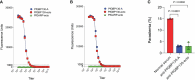
Methods and results: To discover new vaccine candidates, we used our whole proteome differential screening method and identified PfGBP130 as a parasite protein uniquely recognized by antibodies from children who had developed resistance to P. falciparum infection but not from those who remained susceptible. We formulated PfGBP130 as lipid encapsulated mRNA, DNA plasmid, and recombinant protein-based immunogens and evaluated the efficacy of murine polyclonal anti-PfGBP130 antisera to inhibit parasite growth in vitro. Immunization of mice with PfGBP130-A (aa 111-374), the region identified in our differential screen, formulated as a DNA plasmid or lipid encapsulated mRNA, but not as a recombinant protein, induced antibodies that inhibited RBC invasion in vitro. mRNA encoding the full ectodomain of PfGBP130 (aa 89-824) also generated parasite growth-inhibitory antibodies.
Conclusion: We are currently advancing PfGBP130-A formulated as a lipid-encapsulated mRNA for efficacy evaluation in non-human primates.
Keywords: Plasmodium falciparum; blood stage malaria antigen; growth inhibiting activity; mRNA; malaria; vaccine.
Copyright © 2024 Johnson, Shakri, Pond-Tor, Jnawali, Najrana, Wu, Badhai, Alameh, Weissman, Kabyemela, Duffy, Fried, Kurtis and Raj.
Conflict of interest statement
Author JK is a scientific co-founder of Ocean Biomedical which seeks to develop malaria vaccines. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria.Nature. 2020 Jun;582(7810):104-108. doi: 10.1038/s41586-020-2220-1. Epub 2020 Apr 22. Nature. 2020. PMID: 32427965 Free PMC article.
-
Immunogenicity of a recombinant malaria vaccine candidate, domain I+II of AMA-1 ectodomain, from Indian P. falciparum alleles.Vaccine. 2008 Aug 18;26(35):4526-35. doi: 10.1016/j.vaccine.2008.06.031. Epub 2008 Jun 30. Vaccine. 2008. PMID: 18590786
-
The antibody response to Plasmodium falciparum Merozoite Surface Protein 4: comparative assessment of specificity and growth inhibitory antibody activity to infection-acquired and immunization-induced epitopes.Malar J. 2011 Sep 16;10:266. doi: 10.1186/1475-2875-10-266. Malar J. 2011. PMID: 21920045 Free PMC article.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
-
Antibodies and Plasmodium falciparum merozoites.Trends Parasitol. 2001 Apr;17(4):194-7. doi: 10.1016/s1471-4922(00)01946-2. Trends Parasitol. 2001. PMID: 11282510 Review.
Cited by
-
An mRNA Vaccine Expressing Blood-Stage Malaria Antigens Induces Complete Protection Against Lethal Plasmodium yoelii.Vaccines (Basel). 2025 Jun 28;13(7):702. doi: 10.3390/vaccines13070702. Vaccines (Basel). 2025. PMID: 40733679 Free PMC article.
References
-
- WHO . The world malaria report 2021. Geneva: WHO; (2021).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

