Emergence of the brain-border immune niches and their contribution to the development of neurodegenerative diseases
- PMID: 38863704
- PMCID: PMC11165048
- DOI: 10.3389/fimmu.2024.1380063
Emergence of the brain-border immune niches and their contribution to the development of neurodegenerative diseases
Abstract
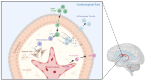
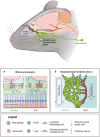
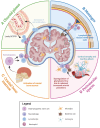
Historically, the central nervous system (CNS) was regarded as 'immune-privileged', possessing its own distinct immune cell population. This immune privilege was thought to be established by a tight blood-brain barrier (BBB) and blood-cerebrospinal-fluid barrier (BCSFB), which prevented the crossing of peripheral immune cells and their secreted factors into the CNS parenchyma. However, recent studies have revealed the presence of peripheral immune cells in proximity to various brain-border niches such as the choroid plexus, cranial bone marrow (CBM), meninges, and perivascular spaces. Furthermore, emerging evidence suggests that peripheral immune cells may be able to infiltrate the brain through these sites and play significant roles in driving neuronal cell death and pathology progression in neurodegenerative disease. Thus, in this review, we explore how the brain-border immune niches may contribute to the pathogenesis of neurodegenerative disorders such as Alzheimer's disease (AD), Parkinson's disease (PD), and multiple sclerosis (MS). We then discuss several emerging options for harnessing the neuroimmune potential of these niches to improve the prognosis and treatment of these debilitative disorders using novel insights from recent studies.
Keywords: Alzheimer’s disease; Parkinson’s disease; brain-border; multiple sclerosis; neurodegeneration; neuroimmune.
Copyright © 2024 Tan, Cunliffe, Hogan, Yeo, Oh, Jin, Kang, Park, Kwon, Kim and Jung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ehrlich P. Das Sauerstoff-Bedurfnisdes Organismus: eine farbenanalytischeStudie. Berlin: Hirschward. (1885).
-
- Bield A, Kraus R. U˙ber eine bisherunbekannte toxische Wirkung der Gal-lensauren auf das Zentralnervensystem. Zhl Inn Med. (1898) 19:1185–200.
-
- Lewandowsky M. Zur lehre der ze-rebrospinalflussigkeit. Z Klin Med. (1900) 40:480–4.
-
- Goldmann EE. Die aussere und innere Sekretion des gesunden und kranken Organismus im Liche der “vitalen Farbung”. Beitr Klin Chir. (1909) 64:192–265.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

