Interactions between cancer-associated fibroblasts and T-cells: functional crosstalk with targeting and biomarker potential
- PMID: 38863724
- PMCID: PMC11165253
- DOI: 10.48101/ujms.v129.10710
Interactions between cancer-associated fibroblasts and T-cells: functional crosstalk with targeting and biomarker potential
Abstract
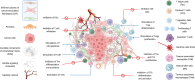
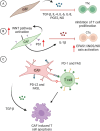
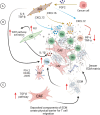
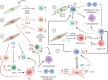
Cancer-associated fibroblasts (CAFs) are a heterogeneous cell population recognized as a key component of the tumour microenvironment (TME). Cancer-associated fibroblasts are known to play an important role in maintaining and remodelling the extracellular matrix (ECM) in the tumour stroma, supporting cancer progression and inhibiting the immune system's response against cancer cells. This review aims to summarize the immunomodulatory roles of CAFs, particularly focussing on their T-cell suppressive effects. Cancer-associated fibroblasts have several ways by which they can affect the tumour's immune microenvironment (TIME). For example, their interactions with macrophages and dendritic cells (DCs) create an immunosuppressive milieu that can indirectly affect T-cell anticancer immunity and enable immune evasion. In addition, a number of recent studies have confirmed CAF-mediated direct suppressive effects on T-cell anticancer capacity through ECM remodelling, promoting the expression of immune checkpoints, cytokine secretion and the release of extracellular vesicles. The consequential impact of CAFs on T-cell function is then reflected in affecting T-cell proliferation and apoptosis, migration and infiltration, differentiation and exhaustion. Emerging evidence highlights the existence of specific CAF subsets with distinct capabilities to modulate the immune landscape of TME in various cancers, suggesting the possibility of their exploitation as possible prognostic biomarkers and therapeutic targets.
Keywords: Cancer associated fibroblasts (CAF); T-cells; tumour immunity; tumour microenvironment (TME).
© 2024 The Author(s). Published by Upsala Medical Society.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives.Mol Cancer. 2021 Oct 11;20(1):131. doi: 10.1186/s12943-021-01428-1. Mol Cancer. 2021. PMID: 34635121 Free PMC article. Review.
-
Interplay between cancer-associated fibroblasts and dendritic cells: implications for tumor immunity.Front Immunol. 2025 May 16;16:1515390. doi: 10.3389/fimmu.2025.1515390. eCollection 2025. Front Immunol. 2025. PMID: 40453074 Free PMC article. Review.
-
CAF-induced physical constraints controlling T cell state and localization in solid tumours.Nat Rev Cancer. 2024 Oct;24(10):676-693. doi: 10.1038/s41568-024-00740-4. Epub 2024 Sep 9. Nat Rev Cancer. 2024. PMID: 39251836 Review.
-
Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages.Oncotarget. 2017 Jan 31;8(5):8633-8647. doi: 10.18632/oncotarget.14374. Oncotarget. 2017. PMID: 28052009 Free PMC article.
-
Cancer-associated fibroblasts in clear cell renal cell carcinoma: functional heterogeneity, tumor microenvironment crosstalk, and therapeutic opportunities.Front Immunol. 2025 Jun 4;16:1617968. doi: 10.3389/fimmu.2025.1617968. eCollection 2025. Front Immunol. 2025. PMID: 40534854 Free PMC article. Review.
Cited by
-
Clinical efficacy and biomarkers of neoadjuvant chemoimmunotherapy in locally advanced esophageal squamous cell carcinoma.Cancer Immunol Immunother. 2025 Jun 18;74(8):243. doi: 10.1007/s00262-025-04099-9. Cancer Immunol Immunother. 2025. PMID: 40531216 Free PMC article.
-
Special issue: frontiers in recent advances on cancer diagnosis and treatment.Ups J Med Sci. 2024 Dec 31;129. doi: 10.48101/ujms.v129.11919. eCollection 2024. Ups J Med Sci. 2024. PMID: 39780954 Free PMC article. No abstract available.
-
Ligustilide Inhibits the PI3K/AKT Signalling Pathway and Suppresses Cholangiocarcinoma Cell Proliferation, Migration, and Invasion.Recent Pat Anticancer Drug Discov. 2025;20(2):200-212. doi: 10.2174/0115748928332384240812060751. Recent Pat Anticancer Drug Discov. 2025. PMID: 39171465
-
Targeting esophageal carcinoma: molecular mechanisms and clinical studies.MedComm (2020). 2024 Oct 15;5(11):e782. doi: 10.1002/mco2.782. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39415846 Free PMC article. Review.
-
Deciphering T-cell exhaustion in the tumor microenvironment: paving the way for innovative solid tumor therapies.Front Immunol. 2025 Apr 1;16:1548234. doi: 10.3389/fimmu.2025.1548234. eCollection 2025. Front Immunol. 2025. PMID: 40236693 Free PMC article. Review.
References
-
- Tarin D, Croft CB. Ultrastructural features of wound healing in mouse skin. J Anat. 1969;105:189–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
