Therapeutic targeting of TP53 nonsense mutations in cancer
- PMID: 38863730
- PMCID: PMC11165251
- DOI: 10.48101/ujms.v129.10719
Therapeutic targeting of TP53 nonsense mutations in cancer
Abstract
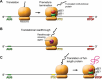
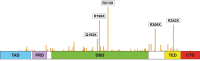
Mutations in the TP53 tumor suppressor gene occur with high prevalence in a wide range of human tumors. A significant fraction of these mutations (around 10%) are nonsense mutations, creating a premature termination codon (PTC) that leads to the expression of truncated inactive p53 protein. Induction of translational readthrough across a PTC in nonsense mutant TP53 allows the production of full-length protein and potentially restoration of normal p53 function. Aminoglycoside antibiotics and a number of novel compounds have been shown to induce full-length p53 in tumor cells carrying various TP53 nonsense mutations. Full-length p53 protein generated by translational readthrough retains the capacity to transactivate p53 target genes and trigger tumor cell death. These findings raise hopes for efficient therapy of TP53 nonsense mutant tumors in the future.
Keywords: TP53; cancer therapy; nonsense mutations; translational readthrough.
© 2024 The Author(s). Published by Upsala Medical Society.
Conflict of interest statement
KGW is co-founder and shareholder of Aprea Therapeutics, a company that develops p53-based cancer therapy including APR-246. Research in the KGW lab has previously received financial support from Aprea Therapeutics. KGW has previously received a salary from Aprea Therapeutics.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
